Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2020.01
| Attachment | Size |
|---|---|
| 905.44 KB |
I. Introduction
The exocrine pancreas is regulated by nerves, mainly the vagus, and gastrointestinal hormones, especially cholecystokinin (CCK) and secretin. A third specific source of regulation is by peptide hormones from the Islets of Langerhans. Islet hormones are relevant because of structural relations within the pancreas. Except for some fishes which have a single larger collection of endocrine cells, islets are dispersed within the pancreas and have the ability to regulate exocrine cells both by local diffusion which manifests as peri-insular halos of acinar cells and by a specific vascular relationship, the islet-acinar portal system. The latter results in acinar cells being exposed to high concentrations of islet hormones and other regulatory molecules. Of these the best defined relationship is with insulin produced in islet beta cells which serves as a trophic factor for the exocrine pancreas, as a short-term signal promoting digestive enzyme synthesis at the translational level, and as part of a long-term control system where dietary carbohydrate leads to the synthesis of the carbohydrate targeted digestive enzyme, pancreatic amylase. Insulin also facilitates digestive enzyme secretion and regulates the membrane transport of glucose and Ca2+. Earlier reviews of this topic include (12, 275, 330). Evidence for a direct effect of other islet peptides is less clear and some may exert an effect through regulating insulin secretion.
II. Structure and Vascular Supply of the Pancreas
A. Structure and distribution of Islets of the Pancreas
Pancreatic islets are collections of endocrine cells scattered through the exocrine pancreas making up 2-3% of the gland. Islets are made up of five types of endocrine cells known as α, β, δ, ε, and PP/F cells that synthesize and secrete, respectively, glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide, all of which are considered peptide hormones. Beta cells are the most numerous cell type (60-70%) and form the core in rodent islets but are scattered throughout the human islet. Alpha cells make up about 20% and along with δ cells (1-2%) form a mantle on the outside of rodent islets. Some of the cell types are geographically distributed with PP/F cells primarily in the ventral lobe of the pancreas. By contrast, ε cells are developmentally regulated making up about 10% of the fetal islet but then differentiate into α and β cells so that in the adult they are rare. Some of the endocrine cells also express a second biologically active peptide (12). Amylin and galanin are synthesized in β cells and are co-secreted with insulin. Pancreastatin is present in α, β, and δ cells where it is derived from chromogranin, and adrenomedulin is produced in PP/F cells. In understanding the effects of these islet peptides on the exocrine pancreas it is important to realize that some (insulin, glucagon and PP) are essentially unique to the islets but others like somatostatin are more broadly distributed in the GI tract, which could affect the exocrine pancreas locally or from systemic sources. Because islet peptides can regulate the other cells within the islet, effects of a particular peptide could be mediated directly on exocrine cells or indirectly by affecting the secretion of insulin or another islet peptide. Furthermore, some of these peptides can affect the CNS and vagal regulation of the exocrine pancreas. Receptors on exocrine pancreatic cells along with direct in vitro effects of islet peptides have been identified for insulin and somatostatin but not for glucagon or PP. Further details with references will be given in the sections on individual peptides.
B. Islet – acinar portal system
Blood flow to the pancreas has been reported to be 0.4 to 1.1 ml per g weight per min with most data from rats, rabbits, mice and dogs (288). Early studies injecting dye or ink into arteries feeding the pancreas showed rapid and intense perfusion of glomerular structures that were identified as Islets of Langerhans (68, 116, 198). Islet blood flow has been most often quantitated using microspheres. Such studies show that 5-20% of blood flow goes to the islets which have a relative blood flow 5-10 times higher than the exocrine pancreas (130, 177). The regulation of blood flow to the two portions of the pancreas is distinct with exocrine secretagogues such as CCK and VIP increasing blood flow to the exocrine pancreas (54, 129) and glucose increasing islet blood flow (131). In a study in rabbits, Lifson and colleagues concluded that essentially all blood flow to the islets goes subsequently to the acinar capillaries (175). Considerable attention has also been paid to the pathway of capillaries within the islets as it pertains to which islet cells are upstream of the others (31, 183).
Early dye injection vascular perfusion studies also showed that small capillary vessels exited the islets and carried blood to the exocrine pancreas and these have been named the insulo – acinar portal system. This directional flow is facilitated by the fact that islet capillaries are slightly larger in diameter which should lead to higher capillary pressure (117, 314). In some species, there are also direct arterioles feeding the acinar tissue and venules draining the islets so not all islet effluent passes to the exocrine tissue. Similar results have been seen for rabbits, rats, mice, guinea pigs, cats, dogs and baboons (115). Evidence for the portal system also comes from scanning electron microscopy of vascular casts and from functional studies. Vascular casts are made by injecting resin or acrylate into the arteries and after polymerization the residual tissue is digested away leaving a three dimensional vascular cast which is coated with gold and viewed in a scanning electron microscope. Each islet has one or sometimes two afferent vessels which break down into a glomus-like capillary network. Efferent vessels similar in size to capillaries leave the islet and connect to the acinar capillaries (82, 83, 216, 225, 341). Similar findings have been made in the rat, rabbit, monkey, horse, baboon and human. Some controversy exists over the extent of this phenomenon especially in rats where Bonner-Weir reported that a large extent of islet blood was drained directly into venules (24) but this was subsequently disputed (204). Lifson and Lassa also described an acinar-ductal portal system in the rabbit pancreas (176). In any case, all blood from exocrine pancreas exits in venules which combine to form the pancreatic veins.
A different type of evidence for the islet-acinar portal system comes from physiological studies particularly using the perfused rat pancreas. Insulin added to the vascular perfusate enhances the exocrine response to CCK and secretin (109, 137, 257, 258). Glucose, which by itself does not affect exocrine secretion, when added to the vascular perfusate enhances the release of insulin and potentiates exocrine secretion (89, 258). Other sugars that do not stimulate insulin secretion had no effect, and epinephrine, which blocks insulin secretion, blocked the potentiating effect of glucose. Since insulin can only come from the islets and would be carried away in the venous drainage in the single pass perfusion system, an effect on exocrine secretion can only come from endogenous insulin reaching exocrine cells by the portal system. Similar studies depleting endogenous somatostatin with cysteamine have also provided evidence for endogenous somatostatin acting through the portal system (238).
Based on the presence of the insulo-acinar portal system, an important question is what is the concentration of islet peptides in the exocrine interstitial compartment. Two estimates indicate that the concentration of insulin and somatostatin is in the nanomolar range. The first comes from a study evaluating saturable binding of iodinated tracer for insulin and somatostatin in the rat pancreas perfused anterograde or retrograde (219). Binding was higher for retrograde perfusion which was attributed to endogenous hormone released during anterograde perfusion. Displacement curves during retrograde perfusion indicated an interstitial concentration of insulin during anterograde perfusion with glucose of 7.5 nM and for somatostatin of 1.1 nM. A second study used a microdialysis technique which showed a unstimulated concentration of insulin of 0.4 nM during both retrograde and anterograde perfusion of dog pancreas (218). The response to stimulation with glucose plus arginine was markedly less during retrograde perfusion. Although the stimulated concentrations did not reach steady state, if insulin release went up 10 fold the interstitial exocrine concentration during glucose stimulation would be about 4 nM. Unfortunately, reported plasma insulin levels in fasting rats and dogs are quite variable with most ranging from 50-400 pM. Much better data is available for humans where most fasting levels are reported to be 25-150 pM.
In summary, although the islet-acinar portal system does not account for all blood flow to exocrine pancreas cells, it does allow a significant fraction of exocrine acinar and duct cells to be exposed to higher concentrations of islet hormones than is the case for other organs. In addition, islet hormones can act directly on acinar cells via specific receptors or indirectly by affecting the release of other islet hormones.
III. Action of insulin on the exocrine pancreas
Insulin appears to be the most important of the classical islet hormones as a regulator of the exocrine pancreas and especially acinar cells. Evidence exists from both human studies and animal models and includes both physiology and disease. Experimental studies have been carried out at different levels of integration from the intact organism to cellular and molecular studies of acinar cells.
A. Clinical studies of the effects of diabetes on the exocrine pancreas
For over one hundred years the pancreas of diabetic patients has been known to be smaller with increased fibrosis consistent with pathology of the entire pancreas and not just the islets (36). Diabetes is now divided into Type 1 or insulin dependent diabetes mellitus (IDDM) where destruction of beta cells leads to lack of insulin, Type 2 whose hallmark is insulin resistance and often does not require additional insulin, and most recently Type 3 which is due to exocrine pathology such as chronic pancreatitis. It has long been recognized that Type 1 and 2 patients may also suffer exocrine abnormalities ranging from subclinical to pancreatic exocrine insufficiency (PEI).
1. Loss of pancreatic mass in diabetes
Autopsy studies of Type 1 diabetes have shown decreased pancreatic weight and volume (91, 184). Methods have been developed to use CT and MRI imaging to determine pancreatic volume and distinguish fat within the pancreas. Ultrasound has also been used but is not as quantitative and is more operator dependent. Studies of diabetic patients with CT and MRI have consistently shown a decrease in pancreatic volume of 30-55% in Type 1 and 15-30% in Type 2 diabetes (96, 178, 188, 242, 323). Some studies have shown a dependence on the duration of diabetes but others have not. Several studies in which type 3 diabetes or a history of alcoholism have been omitted have shown a smaller loss in pancreatic volume. These changes have been confirmed in a recent meta-analysis of 17 studies (88). The authors noted, however, that many of the individual studies were small and classified as low to moderate quality data. Only type 2 diabetes has shown increased pancreatic fat which reduces the volume of parenchymal tissue from the total volume (178, 188). In some studies, with functional as well as imaging data, the decreased pancreatic volume correlated with decreased function as measured by fecal elastase (242). To date there are no longitudinal studies although this could be done with MRI since there is no ionizing radiation.
2. Effects of diabetes on pancreatic function
Direct measurement of pancreatic juice bicarbonate ion and digestive enzymes in response to secretin and CCK using multi-lumen tube collection has been applied to diabetes. Ewald and Hardt list 9 such studies from 1943 to 1996 (73). These studies all show decreased exocrine pancreatic secretion with a bigger effect seen in Type 1 diabetes. An effect of the duration of diabetes was seen in some (162) but not in other studies (80). Most studies show a bigger effect on amylase than on other digestive enzymes and the smallest effect is on bicarbonate.
Indirect pancreatic function testing on stool samples have allowed study of greater number of patients. The most commonly used test is fecal elastase measured by an ELISA assay where <200 µg/g is considered evidence for pancreatic exocrine insufficiency (PEI) and <100 µg/g severe deficiency. Elastase is used because it is more resistant to protease digestion although some studies have measured fecal trypsin or chymotrypsin activity which yields similar results relative to controls. In the first large study of more than 1000 diabetics, 40.7% showed fecal elastase levels of less than 200 µg/g with 22.9% being <100 µg/g (108). Other studies have reported similar results (74, 163, 222) although the frequency of PEI is lower when patients with Type 3 diabetes were excluded (303, 316). Type 1 diabetics with reduced fecal elastase show a higher frequency of steatorrhea up to 60% although the amount of stool fat is modest (35, 107) and clinical PEI requiring enzyme supplementation is rare (38).
Overall, diabetes is accompanied by decreased pancreatic mass and reduced secretion of digestive enzymes along with a diffuse pancreatic fibrosis characterized by intra-acinar fibrosis whose pattern is distinct from that of chronic pancreatitis. These changes may be the result of reduced number of acinar cells, loss of insulin as a trophic factor or alteration in neural control (207). These authors proposed that these changes be recognized as distinct from chronic pancreatitis and be termed “diabetic exocrine pancreatopathy”.
B. Animal studies of the effects of insulin and diabetes on the exocrine pancreas
1. Insulin receptors in the exocrine pancreas
Insulin receptors (IR) on pancreatic acinar cells were first demonstrated by the binding of 125I-insulin in a saturable manner to isolated acini from mouse, rat and guinea pig (156, 263, 282). Scatchard-plot analysis of binding were biphasic and showed high affinity sites with a Kd of about 1.5 nM and capacity of about 10,000 receptors per cell; low affinity sites were much more numerous with a Kd of 88 nM for mouse and 998 nM for rat. Addition of unlabeled insulin accelerated dissociation from rat acini consistent with negative cooperativity rather than two distinct sites (263). The receptor bound insulin analogs with varying potency (insulin > desdipeptide insulin > proinsulin > desoctapeptide insulin) that was similar to the ability of the analogs to increase protein synthesis in acini from diabetic rats (154). Guinea pig insulin is significantly different from other species and guinea pig acinar receptors bound bovine insulin with a higher affinity than guinea pig insulin or porcine proinsulin (282). Mouse acini were also shown to possess distinct receptors for IGF-I and IGF-II (328).
The localization of insulin binding to acinar and other cells in the pancreas has been demonstrated by light and EM autoradiography. When 125I-Insulin was injected intravenously, saturable uptake was observed by the pancreas (19, 46, 261). EM autoradiographs showed silver grains localized both over the plasma membrane and intracellularly in acinar cells. Saturable binding was also observed over duct cells (261). In an in vitro study of 125I-Insulin binding to rat acini, silver grains from 125I were primarily over the plasma membrane at 3 min but by 30 min were primarily intracellular (97). Studies in other cell types have shown that both insulin and its receptor are internalized although the function of internalized insulin is unclear.
Insulin receptors on acinar cells are also subject to regulation. Insulin down regulates its own receptor in a variety of cells, including acinar cells, when they are exposed to high concentrations of insulin through the islet-acinar portal blood system (12, 324). Acini prepared from diabetic mice showed an increased number of IRs compared to normal mice and addition of insulin to 24 hour cultured acini decreased insulin binding (209). Using pancreatic AR42J cells, insulin decreased the biosynthesis of insulin receptors in part by decreasing IR mRNA levels (209, 226). The binding of insulin was also affected by CCK and other secretagogues (228). CCK, carbamylcholine, active phorbol ester and calcium ionophore (A23187) all decreased high affinity binding without affecting insulin internalization or degradation.
The occupancy of insulin receptors has also been related to the biological effects of insulin on acinar cells. Insulin increased glucose transport (uptake) by acini from normal and diabetic mice (156, 329) with a greater effect on acini from diabetic animals that was seen at lower concentrations of insulin. This greater effect is due both to lower contaminating insulin levels and IR upregulation. In acini from diabetic rats and mice, insulin increased protein synthesis as shown by incorporation of radioactive amino acids (154). Of likely relevance to protein synthesis, insulin rapidly increased the phosphorylation of ribosomal protein S6 which is associated with increased protein synthesis (33, 290). The concentration dependence and analog specificity of these actions of insulin is similar to the data for receptor occupancy (98).
More recently IR have been demonstrated in pancreatic acini by immunoblotting of the beta subunit of the receptor (265). The subunit had an apparent size of about 95 kDa and was essentially absent after deletion of the IR gene in acinar cells. The biological response to insulin in the pancreas of these mice was greatly reduced
2. Insulin regulation of exocrine pancreas biosynthetic and metabolic effects
Gene expression
Insulin is known to regulate both the amount and activity of a number of anabolic processes and specific metabolic enzymes. In the exocrine pancreas the best studied tissue specific regulation is that of the digestive enzyme amylase which acts on dietary starch and glycogen (325). The pancreatic amylase content is known to decrease by over 90% in experimental diabetes in rats and the fall can be reversed by administration of insulin. This has been studied primarily in rats given alloxan or streptozoticin which induce beta cell death and diabetes (2, 15, 17, 58, 235, 283, 286). At the same time other digestive enzymes including trypsinogen, chymotrypsinogen and lipase increase slightly and ribonuclease decreases slightly. Amylase also decreases in mouse pancreas in experimental diabetes but by only about 50%. Pancreatic amylase also decreases in the Zucker fatty rat and the ob/ob mouse, well characterized models of insulin resistance (304).
The decrease in pancreatic amylase in experimental diabetes is accompanied by a decrease in amylase synthesis (286). Moreover, insulin increased amylase biosynthesis in rat pancreatic derived AR42J cells (210). The major cause of the decreased amylase synthesis and tissue content is a decrease in amylase mRNA which was first reported by Korc et al (60, 155). The decrease in amylase mRNA could be reversed by insulin. Chymotrypsinogen mRNA showed a small change in the opposite direction and there was essentially no change in salivary amylase or its mRNA. Subsequently, Brannon and colleagues showed that dietary glucose and insulin together regulated pancreatic amylase (Amy2) gene expression (306). More detailed information on the mechanism of amylase gene regulation comes from studies in mice. A 30-bp region in the proximal amylase promoter overlapping with the PTF1 binding site present in most pancreatic digestive enzymes contains the insulin-dependent element and can transfer insulin sensitivity to the elastase promoter (134, 140). This regulatory region is not present in all mouse amylase alleles and this can explain why some mouse strains are not as sensitive to diabetes and insulin (57). Unfortunately, there has been little subsequent work defining how insulin receptor signaling regulates the insulin response element.
Membrane transport
Insulin is known to stimulate membrane transport of many substances usually by effects on the amount of or properties of specific transport proteins. The best studied and probably the most important physiologically is the uptake of glucose into fat and muscle cells that is mediated by the glucose transporter, GLUT4. GLUT4 is present at rest in intracellular vesicles that translocate to the plasma membrane in response to insulin, increasing glucose uptake 5-20 fold. Most other cell types contain GLUT1 that is regulated by another mechanism but to a lesser extent. In mouse pancreatic acini, insulin stimulated the uptake of the nonmetabolizable sugar 2-deoxy-glucose (2DG) about two fold (156). A more robust effect of lower concentrations of insulin was seen when acini were prepared from diabetic mice and uptake of both 2DG and 3-O-methyl glucose, a non metabolizable glucose analog, were increased (329). Glucose uptake was not affected by inhibitors of protein synthesis but was reduced by the actin inhibitor, cytochalasin B.
In some tissues insulin also stimulates the uptake of amino acids through effects on specific amino acid transporters. Uptake of amino acids have been studied in many pancreatic preparations. One of the earliest studies using microdisected pieces of mouse pancreas showed that amino acids were taken up and oxidized in preference to glucose (48). Neither CCK or insulin affected uptake and transport did not appear to be separated from protein synthesis. Latter studies used perfused pancreas or isolated acini and showed that the basolateral membranes of pancreatic acinar cells possess 4-5 distinct transporters as measured by physiological studies or gene expression (190, 252). Insulin stimulation studies in the perfused rat pancreas show effects of exogenous insulin and diabetes on two Na+-independent transporters, the Asc transporter used by serine (193, 221) and a basic amino acid transporter termed y+ for lysine/arginine (215) while no effect was seen on the Na+ dependent dependent transporters such as the system A transporter for AIB and glutamine (192). Moreover, increasing glucose in the perfusate which released endogenous insulin had no effect (221). Studies in isolated rat pancreatic acini also showed no effect of insulin on AIB and cycloleucine uptake which are not used for protein synthesis although it stimulated incorporation of leucine into protein (154). In a more recent study of the mouse pancreas, Rooman et al analized the expression of 37 genes encoding transporters and found expression of a number with the highest expression of slc7a8 and slc3a2 and confirmed the expression of five by western blot (252).
Protein synthesis
Insulin is known to stimulate protein synthesis by translational effects in many tissues (132). Early studies in the pancreas involved in vivo studies of the incorporation of radioactive amino acids into total protein or specific enzymes of normal and diabetic rodents (45, 61, 157). While most of the studies showed a positive effect of insulin the results are complicated by changes in the precursor pool or changes in the levels of mRNAs. When protein synthesis in mice was quantitated by autoradiography, there was more incorporation into peri-insular acinar cells then into tele-insular acinar cells (7). This difference was lost following treatment with streptozotocin and the authors ascribed it to insulin. One study overcame some of these issues by changing plasma insulin and glucose in vivo but measuring protein synthesis in vitro using pancreatic lobules incubated in a constant medium (2). It showed that either glucose infusion in vivo or treatment with a sulfonylurea drug both of which stimulate insulin release increased protein synthesis by 25 – 40%. When Zucker fatty rats were studied as a model of insulin resistance, overall pancreatic protein synthesis was reduced by nearly 50% (305). There was considerable difference in synthesis between different digestive enzymes separated by 2-D-gel electrophoresis. A morphological study of prolonged diabetes in rats showed gross abnormality in the secretory pathway 28 days after STZ treatment that was partially reversed by insulin administration (333). However, shorter studies have not shown significant structural alterations 1 week after STZ other than the appearance of cytoplasmic lipid droplets (154).
More recent studies have been able to overcome some of the methodological issues by measuring the effects of insulin over short times to prevent changes in mRNA and under conditions where the precursor pool is large and constant (264). In vitro studies have used isolated pancreatic acini under dilute incubation to keep the precursor pool constant. Insulin stimulates the incorporation of multiple different amino acids (leucine, methionine, phenylalanine) in isolated acini from diabetic rats (153, 154, 160, 227). Similarly, insulin increased methionine incorporation into total protein and immunoprecipitated amylase in rat pancreatic derived AR42J cells (210). The effect of insulin on both cell types occurred over concentrations from 30 pM to 100 nM and was mediated by the insulin receptor as insulin analogs stimulated in parallel to their ability to bind to the insulin receptor (154, 227). Insulin was also shown to have nonparallel effects on different digestive enzymes and structural proteins such as myosin and LDH (227).
Although the mechanism of insulin signaling is well studied in a number of target tissues especially liver, fat and muscle, only a few studies have been carried out in the exocrine pancreas. The main signaling pathway regulating protein synthesis is the mTOR pathway and this pathway has been documented in pancreatic acinar cells primarily in mediating the actions of CCK and acetylcholine to stimulate digestive enzyme synthesis (266, 326). Insulin has been shown to activate S6 kinase and S6 phosphorylation downstream of TORC1 in rat and mouse acini (33, 290). Akt is upstream of mTOR and insulin activates the phosphorylation of Akt on S473 and T308 in rat acini (20). This action appeared mediated by PI3K. That these actions are important physiologically is shown by over a 50% reduction in mTOR pathway activation after feeding in mice without acinar cell insulin receptors (265).
Secretion
The effects of insulin administration and diabetes on pancreatic exocrine secretion have been studied in a number of species and systems ranging from intact awake animals down to isolated acinar cells. The more isolated systems have better defined parameters but lack neural control and other integrative aspects found in the intact organism.
In vivo studies have largely been carried out in rats with some studies in dogs and other species and often have compared streptozotocin (STZ) treated and intact animals. STZ and alloxan treatment damage islets thus lowering insulin levels and leading to hyperglycemia. In STZ-diabetic rats pancreatic juice flow has been reported to be moderately increased (18, 241, 285). In two studies this effect was reversed by transplanting syngeneic islets into the liver (53, 168). However, in mice STZ treatment or genetic models of diabetes leads to reduced juice flow (265), and in sheep, alloxan treatment led to a substantial decrease that could be restored by insulin treatment (243). In all of these studies the effects of the diabetic state on plasma glucose and lipids is not clear. The effects of CCK on digestive enzyme secretion in diabetes are complicated by changes in the pancreatic content of specific digestive enzymes. The concentration of secreted amylase is decreased and trypsin increased although both respond to CCK (18, 285). The most valid results may be in animals with chronic pancreatic fistulas where juice can be collected in the unanaesthetized state but such studies have not been carried out in diabetes.
Other studies have focused on the effects of insulin in normal animals with intact islets. When a high concentration of insulin (4 U per 100 gm) was injected into the femoral vein, subsequent pancreatic stimulation by CCK was potentiated (137). In another study, insulin (1U as a bolus) induced hypoglycemia, increased fluid flow and increased amylase and total protein output; this effect was blocked by atropine and ascribed to hypoglycemia-induced vagal cholinergic activation (241). In a third study an intravenous infusion of insulin also increased pancreatic secretion (76). Not all studies however have reported this effect (284). In a different approach using unanaesthetized rats, IV injection of rabbit anti-insulin serum but not control serum blocked the pancreatic response to a liquid meal and to exogenous CCK or secretin stimulation (167). In a study in conscious dogs which secrete a high bicarbonate pancreatic juice, most of which comes from the ducts, insulin administration diminished secretin stimulated bicarbonate secretion and this effect was blocked by prior pancreatic denervation (21). This study illustrates the complexity of administering insulin in vivo and show that some of the effects of high dose insulin in vivo are mediated by neural pathways. However, overall they suggest a positive effect of insulin on pancreatic exocrine secretion.
Studying an in vitro pancreas preparation removes the effects of plasma glucose and the brain initiated neural control. While some studies have been carried out with isolated pancreas segments from animals with thin pancreas, the perfused pancreas provides better oxygenation and when single pass perfusion is used the secretion of both endocrine and exocrine secretions can be separately measured. Because the islets are present in their normal position, they can be stimulated independent of the exocrine pancreas and the islet hormones will reach the exocrine cells by local diffusion and even more importantly by the islet-acinar portal system. Most studies of the perfused normal rat pancreas or isolated pancreatic segments incubated with normal glucose have shown that exogenous insulin has little or no effect on basal fluid and protein secretion but potentiates the secretory effect of CCK, ACh, or secretin (89, 109, 135, 137, 169, 240, 257, 258, 277). In STZ induced diabetic animals with high plasma glucose, the in vitro response to secretin, and CCK was reduced compared to normal rats (229, 230). This change could be partially reversed by treatment with insulin. To study the effect of endogenous insulin, investigators have used a low plasma glucose background and then a pulse of high glucose to stimulate insulin release; this endogenous insulin which must reach the acinar cells by the islet-acinar portal system increased secretion of fluid, protein and amylase in response to CCK or ACh (89, 257, 258). This effect was induced only by sugars that stimulate insulin secretion and could be blocked with epinephrine or somatostatin which block glucose induced insulin release. In an alternative approach, Chey and colleagues added anti-insulin antibody to the perfusate in the rat or dog perfused pancreas and showed that this resulted in reduced juice, bicarbonate and protein secretion (165-167). All of this data is consistent with insulin having an action on acinar and duct cells to facilitate secretion. However, other studies have reported that both high glucose and exogenous insulin inhibits CCK stimulated amylase secretion in the mouse (47) or caerulein-stimulated perfused rat pancreas (32). In the perfused cat pancreas, insulin had no effect on the basal fluid or enzyme secretion (332). While a stimulatory effect of insulin on acinar cells is consistent with the presence of insulin receptors on acinar and duct cells and the ability of insulin to act on isolated pancreatic acini (see below), other studies have suggested effects within the islet can explain much of the actions of insulin. In the studies of immunoneutralization of insulin in perfused rat and dog pancreas, Chey’s group showed that insulin antibody increased the concentration of somatostatin and pancreatic polypeptide in the venous effluent. In their rat study, addition of a antisomatostatin antibody partially reversed the effect of the anti-insulin and the addition of somatostatin partially reversed the effect of combined stimulation by CCK and secretin (166, 169). Using a perfused canine pancreas, insulin antiserum blocked secretion but when SS and PP antibodies were added together, the effect on fluid and bicarbonate was fully reversed and the inhibition of protein secretion was partially reversed (165). These investigators concluded that the inhibitory effect of insulin antisera was mediated by the local release of and action on the exocrine pancreas of somatostatin and PP (165). In a related study, Nakagawa et al concluded that the net effect of islet peptides on the exocrine pancreas was negative because exocrine secretion was greater with retrograde than anterograde perfusion (219). Thus the action of insulin could be mediated at least in part by suppressing the secretion of inhibitory islet peptides. One study of duct function showed that insulin increased pancreatic juice secretion and that this effect was blocked by ouabain (109).
Isolated pancreatic acini and ducts can be used to study cellular function in the absence of neural, hormonal and islet control. Isolated acini are exquisitely sensitive to CCK, acetylcholine, and bombesin, and respond through an increase in intracellular Ca2+ to increase digestive enzyme secretion. Acini also respond through an increase in cyclic AMP to secretin and VIP which can increase enzyme secretion or potentiate the actions of agonists that mobilize Ca2+. Isolated acini from STZ induced diabetic rats show reduced secretion of amylase and ribonuclease in response to CCK and cholinergic agonists (233-235, 239). This decreased secretion is also seen with alloxan-induced diabetes and can be reversed by treatment of the animals with insulin. One cause of the decreased secretion is a decrease in the synthesis and content of digestive enzymes. When secretion is normalized to content and expressed as per cent of content released per 30 min, maximal secretion is similar but the dose response to CCK is shifted to the right while the response to carbachol is similar. Thus, there is also an effect on CCK receptors which was confirmed by radioactive binding of CCK showing changes in affinity and capacity of the two affinity states (233). Interestingly, RT-PCR of the CCK1 receptors showed no change in pancreatic receptor mRNA in rat STZ induced diabetes (255). Acinar cell post receptor signaling is also altered in diabetes with changes in CCK-induced IP3 formation (40, 149, 255). This is accompanied by changes in the amount and phosphorylation of IP3 receptors. The increase in intracellular free Ca2+ measured in suspension or single cells is also reduced in diabetes (239, 255).
An effect of insulin in vitro on both diabetic and normal rat acini has also been demonstrated. An effect of insulin to increase amylase release was seen as early as 30 min and increased to a maximum at 2 h (234). Over this time the number and affinity of CCK receptors was also changed. In another study in acini from normal rats, insulin after 90 min potentiated the secretion in response to CCK plus secretin but had no effect on either alone; this potentiating effect of insulin was inhibited by the Na+ - K+ ATPase inhibitor, ouabain (197). Insulin also has been shown to directly activate the plasma membrane Ca2+ ATPase (PMCA) in rat (191).
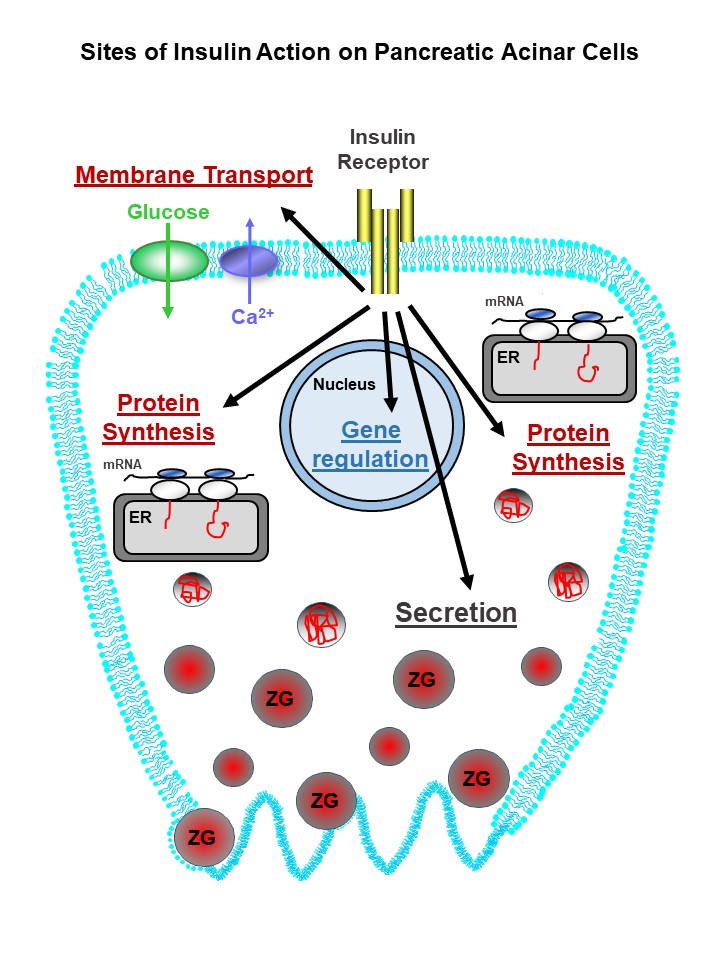
These studies along with other metabolic effects of insulin on isolated acinar cells indicate that insulin can directly affect acinar cell secretion. However, the mechanism of these effects are not fully established. Moreover, all studies have been carried out in rats and rat tissue and the results need to be extended to other species. The cellular sites where insulin acts on pancreatic acinar cells are shown in Figure 1. Finally, data is needed from isolated ducts to establish whether there is a direct effect of insulin on ductal bicarbonate secretion.
Figure 1. Sites of insulin action on pancreatic acinar cells. Insulin has receptor dependent action to regulate protein synthesis at the translational level, gene expression at the transcriptional level, membrane transport of glucose and calcium, and potentiation of digestive enzyme secretion. Not all of the steps between receptor occupancy and the end biological effect are known and most of the data is from rodent pancreas. (See text for details and references).
C. Effects of insulin on pancreatitis and other pancreatic diseases
1. Clinical and animal studies linking diabetes with pancreatitis
There is emerging evidence from clinical studies (93, 99, 106, 202, 250, 271) and animal studies (111, 114, 338) that pre-existing diabetes may pre-dispose, increase the risk or make pancreatitis more severe. This implies that endogenous insulin may have a protective role during pancreatitis. Type-2 diabetics have an ~3 fold increased risk of developing acute pancreatitis (AP) (93, 220, 250, 271), which could be explained by a loss of direct protection of insulin on pancreatic acinar cells, due to insulin resistance. Pre-existing diabetes increases the severity of acute pancreatitis (202) and diabetes increases the mortality in patients with chronic pancreatitis (148, 172). Around half of type-1 diabetic patients exhibit lesions within the exocrine pancreas that are reminiscent of chronic pancreatitis (106).
Moreover, acute pancreatic patients with hyperglycemia are at higher risk of multiple organ failure (199). Hyperglycemia frequently accompanies severe acute pancreatitis and is used in the Ranson score as a predictor of disease severity (246). Furthermore, the incidence of AP is higher among type-2 diabetics compared to the normal population and the risk of AP was reduced among diabetic patients treated with insulin or metformin compared to those treated with other drugs such as sulphonylurea based antidiabetic drugs (99).
For many years there have been numerous anecdotal histological observations of the pancreas in animals that the acinar cells surrounding the islets (peri-islet acinar cells) are morphologically distinct from acinar cells distant from the islets. This was further investigated in L-arginine-induced experimental animal models of pancreatitis in which peri-islet acinar cells remained relatively intact compared to distal injured acinar cells, (111, 113, 114, 296). This peri-islet acinar cell protection was abolished in streptozotocin (STZ)-induced diabetic rats, with impaired insulin secretion (111, 112, 114). These studies suggest that insulin release has a paracrine protective role in the pancreas. Moreover, in addition to the acute injury, the regeneration of exocrine pancreatic tissue was abolished in diabetic rats and restored following the administration of exogenous insulin (111, 113, 114, 296). This was further investigated in the seminal study by Zechener et al 2012 (338), in which caerulein-induced pancreatitis was aggravated in streptozotocin (STZ)-induced type-1 diabetic mice (338). Specifically, they showed that numerous markers of the acute phase of pancreatitis injury (within 24 hours) were markedly potentiated; including plasma amylase, lipase and trypsinogen, pancreatic edema (wet/dry weight ratio), pancreatic histological tissue injury score (H&E), inflammation and cell death. In addition, regeneration of the pancreas (7 days later) was delayed (338). Moreover, this diabetic-induced potentiation of pancreatitis phenotype was partially corrected by exogenous administration of insulin (slow release pellet implant). They also suggested that the mechanism for this more severe pancreatitis phenotype in STZ-induced diabetic mice was due at least in part to depleted pancreatic regenerating islet-derived 3β (REG3β) peptide, which has important anti-inflammatory and antimicrobial properties. REG3β is upregulated during pancreatic injury and is suggested to act as an acute emergency program to circumvent the inflammatory response during pancreatitis (43, 94, 312, 340). However, REG3β expression was found to be severely blunted in the pancreas of STZ-induced diabetic mice, suggesting that this may contribute to the more severe pancreatitis phenotype (338).
2. Insulin protection during cellular models of pancreatitis
From a clinical perspective, and to some extent the diabetic animal studies, it is very difficult to separate the confounding effects of hyperglycemia or reduced systemic effects of insulin from a loss of direct insulin protection of acinar cells. This is important because hyperglycemia is associated with a more severe pancreatitis, by providing a pro-inflammatory environment and by facilitating sepsis (251). Moreover, insulin itself exhibits anti-inflammatory properties (159), therefore the systemic loss of insulin secretion or insulin effectiveness would be predicted to promote systemic inflammation. However, studies using acutely isolated pancreatic acinar cells provide compelling evidence that insulin directly protects acinar cells from cellular injury induced by pancreatitis-inducing agents, such as oxidative stress (H2O2) or the alcohol/fatty acid metabolite, palmitoleic acid (POA) (cellular models of acute pancreatitis) (191, 262). Specifically, insulin markedly attenuated ATP depletion, the inhibition of plasma membrane calcium ATPase (PMCA) which transports Ca2+ out of cells, cytotoxic Ca2+ overload and necrotic cell death. This protection was partially PI3K/Akt-dependent and due to an acute metabolic switch from mitochondrial to glycolytic metabolism (191, 262). Consistent with this, insulin enhanced PMCA inhibition by glycolytic inhibitors and abolished PMCA inhibition by mitochondrial inhibitors (191). Therefore, this switch to glycolysis appears to maintain cytosolic ATP concentration sufficiently to fuel the PMCA and thus prevent cytotoxic Ca2+ overload, even in the face of impaired mitochondrial function.
The most likely downstream signaling pathways responsible for insulin’s protective effects during pancreatitis are likely to be mediated by tyrosine kinase or PI3K/Akt. It’s also interesting to note that several related growth factors (GF), that also couple to tyrosine kinase and PI3K/Akt , similar to insulin, are also reported to be protective in animal models of pancreatitis (50, 318, 319). These include insulin-like growth factor (IGF-1), fibroblast growth (FGF), hepatocyte growth factor (HGF) and epidermal growth factor (EGF). Although, altered expression of signaling proteins and metabolic enzymes may contribute to insulins protection in vivo, most of the protective effects of insulin observed in acinar cells occurred over a relatively short-term (15-30 minutes), suggesting a more rapid effect of post-translational effects, such as tyrosine kinase or PI3K/Akt phosphorylation.
Arguably, the most important mechanism for insulin’s protection is the regulation of glycolytic ATP. Glycolytic flux is primarily regulated by the activity of phosphofructokinase-1 (PFK1), which catalyses the conversion of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6BP) and represents the first rate-limiting irreversible step in glycolysis. Insulin has been shown to lead to the Akt-mediated phosphorylation and direct activation of phosphofructokinase-2 (PFK2); otherwise known as phosphofructokinase-fructose bisphosphatase (PFKFB). PFKFB consists of four separate bi-functional glycolytic enzymes (PFKFB1-4), with varying catalytic and functional activities (51). PFKFB catalyses the conversion of F6P to fructose-2,6-bisphosphate (F2,6BP), via their kinase activity, but also the reverse reaction, by converting F2,6BP back to F6P, via their bisphosphatase activity (51). F2,6BP is a potent positive allosteric activator of PFK-1, which maintains high glycolytic flux (14). Therefore, Akt-mediated phosphorylation of PFKFB2 may represent the major molecular mechanism by which insulin increases glycolytic ATP supply in the face of impaired mitochondrial metabolism during acute pancreatitis.
3. The effect of insulin on the gut microbiome-pancreatitis link
Severe acute pancreatitis is frequently accompanied by infected pancreatic necrosis and systemic bacteremia or sepsis (37). The source of the bacteria is thought to be from “leaky” gut, particularly the colon, which houses a large reservoir of pathogenic and commensal bacteria (317). This caused by gut dysbiosis, inflammation, altered mucus secretion and the consequent loss of gut barrier function and bacterial translocation into the blood (37). The gut microbiome consists of a delicate balance of trillions of bacteria from thousands of different bacterial species that can be broadly categorized into “good” (anti-inflammatory) or “bad” (pro-inflammatory). Moreover, bacteria can aid in the breakdown and absorption of undigested carbohydrates and produce numerous beneficial anti-inflammatory metabolites, such as the short chain fatty acids (SCFAs) butyrate, acetate and proprionate that promote epithelial integrity and gut barrier function (297).
This delicate balance of the gut microbiome and bacterial diversity is maintained by numerous anti-microbial peptides (AMPs) secreted mainly from the Paneth cells within the crypts of Lieberkuhn of the small intestine (8). However, several studies show that AMPs are secreted from pancreatic acinar cells, which may contribute to maintaining a healthy gut microbiome. These include the related C-type lectin family members; lithostathine (REG1α/β) (49), REG3 family members (REG3α/β/γ) - sometimes referred to as pancreatitis-associated peptide (PAP)/ hepatocarcinoma-intestine-pancreas (HIP) (43, 94, 312, 340), cathelicidin-related AMP (CRAMP (4)), and defensins (268).
PAP was originally discovered in pancreas homogenates, secretory granules and in the pancreatic juice during experimentally-induced acute pancreatitis in rats, but was absent in control rats (139). PAP expression was also increased in pancreatic acinar cells in response to both acute and chronic pancreatitis (232). Moreover, PAP/REG3β expression is regulated by dietary carbohydrates (64) and is blunted in diabetic mice (338), suggesting that insulin regulates the expression of PAP/REG3β. Therefore, during pancreatitis the loss of PAP/REG3β secretion into the gut may accentuate the gut dysbiosis, inflammation and loss of barrier function and bacterial translocation, thereby further contributing to the severity of acute pancreatitis.
It is also possible that gut dysbiosis in diabetics may be caused by reduced pancreatic secretion of amylase. Amylase is a highly abundant digestive enzyme that breaks down starch in the gut and its expression and secretion is controlled by insulin and high carbohydrate diet (248) and is reduced in diabetic animals (154). Such loss of amylase secretion may also alter the gut microbiome, due to huge amounts of undigested starch entering the colon leading to overgrowth of bacteria that break down un-digested starch (Bacteroides spp). However, this has never been fully and specifically investigated and it remains unclear whether reduced amylase per se in the gut would be beneficial or detrimental. Even though pancreatic enzyme insufficiency (PEI) leads to numerous co-morbidities that might be explained by an altered gut microbiome, disentangling the specific effects of reduced amylase secretion from the loss of other digestive enzymes would be difficult.
It is also interesting that the gut microbiome is able to signal to and thus contribute to the maintenance of a healthy inflammatory and immune landscape within the pancreas, and when disrupted may ultimately leading to autoimmune type-1 diabetes (289). Specifically, SCFAs, such as butyrate, released from gut bacteria are able to leak into the circulation and reach sufficient concentration to promote the secretion of CRAMP from pancreatic β cells. CRAMP induced a positive immunoregulatory phenotype in pancreatic resident macrophages by promoting the production of anti-inflammatory cytokine, TGFβ, to maintain immune homeostasis via Treg induction. However, the loss of SCFAs following gut dysbiosis disrupts this process, leading to reduced CRAMP secretion which promotes a pro-inflammatory environment in which activated macrophages secrete TNFα and the ultimate autoimmune destruction of pancreatic β cells and the consequent Type-1 diabetes (289). This therefore suggests a complex reciprocal regulation between the gut microbiome, the exocrine and the endocrine pancreas.
4. Insulin as a therapy to treat acute pancreatitis
Insulin therapy has been used to specifically treat hypertriglyceridemia (HTG)-induced pancreatitis with promising outcomes (125, 247). HTG occurs when plasma lipids exceeds 1,000 mg/dL (normal range, 101-150 mg/dL) (182). HTG-induced pancreatitis is relatively rare, accounting for 2.3% to 10% of all cases of acute pancreatitis, but is a well-documented etiological risk factor leading to severe disease (307). The rationale for using insulin therapy to treat HTG-induced pancreatitis is that it lowers plasma triglycerides, by activating lipoprotein lipase (convert triglycerides into free fatty acids) and inhibiting the hormone-sensitive lipase (liberates adipocyte triglyceride), thereby limiting inflammation (66). However, given the evidence presented above, it’s entirely possible that the beneficial effects of insulin therapy in HTG-induced pancreatitis patients could be due to a direct protection of acinar cells.
Insulin is also used as the standard of care for all critical care patients, including those with severe acute pancreatitis, with the aim of targeting hyperglycemia associated with the acute phase of injury, which facilitates inflammation and sepsis (287). There have been numerous clinical trials and meta-analyses testing the effectiveness of intensive insulin therapy in critical care patients and some studies question whether there is any overall patient benefit (101, 181, 322, 334-336). However, this is likely because in patient groups receiving insulin, fewer patients die from septicemia, but more die from the complications of inadvertent hypoglycemia, an independent risk factor of mortality (67, 126, 158, 310).
However, given the direct protective effect of insulin on acinar cells described above there is a strong rationale to test high dose insulin infusion with very tight moment-to-moment glucose control to specifically target the acinar injury. This could be achieved using the hyperinsulinemic euglycemic clamp with automated insulin minipumps combined with continuous closed loop subcutaneous glucose monitoring devices (170). It could be argued that there is a greater requirement for high dose insulin because pancreatic acinar cells receive a portal blood flow (175) with ~10 times higher insulin concentration than the systemic circulation (218, 219). Furthermore, stress hormones, such as adrenaline, cortisol and glucagon, and inflammatory cytokines (TNFα and IL-1β) reduce tissue sensitivity of insulin, so a higher dose of insulin may be necessary to overcome this. Moreover, high dose insulin infusion (8 mU/kg/min; adjusted for surface area to weight ratio) with tight physiological glucose control has been tested in healthy human volunteers during endurance exercise studies with no reported adverse effects (189).
IV. Action of other islet hormones/peptides on the exocrine pancreas
A. Glucagon
Glucagon was originally identified in pancreatic extracts as a hyperglycemic-glycogenolytic factor that came from the α cells of the pancreatic islets (102, 293). Foa and colleagues showed by cross circulation experiments in dogs that hypoglycemia induced by insulin triggered glucagon release which causes hyperglycemia in the recipient animal (78). The subsequent development of a radioimmunoassay for glucagon by Unger made more detailed studies of the physiology and pathophysiology of glucagon possible (309).
Glucagon is a 29 amino acid peptide derived from proglucagon in islet α cells through the tissue specific processing by prohormone convertase 2 (PCSK2) (87). By contrast, in intestinal enteroendocrine cells proglucagon is processed to GLP-1, GLP-2, oxyntomodulin, and glicentin (171). The major role of glucagon is to antagonize the effects of insulin and maintain plasma glucose homeostasis by promoting hepatic gluconeogenesis and glycogenolysis and inhibiting glycogen synthesis (25). The major regulators of glucagon secretion by islet alpha cells are glucose which inhibits and amino acids as well as parasympathetic and sympathetic nerves which stimulate glucagon release. The suppression by high glucose, however, is not direct but mediated by insulin/GABA secreted by islet beta cells. Somatostatin also suppresses glucagon secretion.
The effect of exogenous glucagon on exocrine pancreatic secretion has been studied in vivo with a consistent effect of high concentrations of purified crystalline glucagon to inhibit exocrine secretion of fluid, bicarbonate and protein stimulated by food, CCK or secretin in dogs (65, 123, 152, 276), cats (151), rats (1, 22, 272), and humans (42, 52, 105). The mechanism of this inhibition is unclear in these studies. In vitro, studies have been carried out in the perfused pancreas, pancreatic segments and lobules with mixed results (25). With the development of isolated pancreatic acini and acinar cells, natural purified glucagon was shown to stimulate amylase secretion and increase cyclic AMP in rat, mouse and guinea pig acini (237, 278, 279). However, the stimulatory principle did not elute with synthetic glucagon so its nature is unknown. Most importantly, synthetic glucagon has no effect on amylase secretion by isolated acini (10, 237). Glucagon receptor mRNA has been identified in the pancreas but not in isolated acini or ducts (63, 104). Thus, these studies do not support a direct effect of glucagon on acinar cells. Whether the islets or the nervous system is involved is not yet established.
B. Somatostatin
Somatostatin (SS) was originally identified in hypothalamic extracts as a factor able to inhibit growth hormone release and was subsequently purified as a cyclic peptide containing 14 amino acids (SS-14) (27). A second form extended at the amino terminal and containing 28 amino acids (SS-28) was later characterized (244). Subsequently, somatostatin was found to be widely distributed in the body including other regions of the brain, the small intestine, pancreatic islets and the stomach. For information on the mRNA sequence and the somatostatin precursor see (208). Outside the nervous system, including islets, somatostatin is produced in D cells and is now considered to be both a hormone and a paracrine regulator and generally acts as an inhibitor of specific physiological processes such as gastrin secretion in the stomach and insulin secretion in the islet. The effect of somatostatin is mediated by specific receptors which most often act to inhibit adenylyl cyclase but can also regulate ion channels and activate tyrosine phosphatase (249).
In all species studied plasma somatostatin goes up after a meal; in humans the basal concentration is about 10 pM and this doubles after eating (103, 343). The majority of circulating SS comes from the gut and not the islets (295). However, somatostatin released from islet D cells can have direct effects on other islet cells and pancreatic exocrine cells. Somatostatin release is controlled by cholinergic and adrenergic nerves as well as gastrointestinal hormones including CCK, secretin and VIP (208). The half-life of SS-14 in plasma is 1-2 min while that of SS-28 is 3-4 min (313).
Pancreatic exocrine secretion of fluid and enzymes is inhibited by in vivo administration of SS-14 or SS-28 in conjunction with stimulation by meal feeding or administration of CCK and/or secretin in dogs (23, 138, 147, 291, 292, 331), rats (41, 79), cats (6), rabbits (203), and humans (56, 69, 120). Additional studies using long acting SS analogs such as Octreotide (SMS201-995) and RC160 have shown similar results (141, 150, 194, 294). That an effect of endogenous SS release on the pancreas may have physiological importance is suggested by the fact that exogenous SS mimicking postprandial levels is able to inhibit insulin and amylase secretion (103). Moreover, immunoneutralization of circulating SS leads to an enhancement of CCK stimulated pancreatic amylase secretion (311).
The site at which SS inhibits exocrine pancreatic secretion and its physiological importance is unclear. Suggested sites include the central or peripheral nervous system, within the islet through effects on insulin secretion and at the level of pancreatic exocrine cells. It is of course possible that SS acts at more than one site. Because this review focuses on regulation of exocrine pancreas by islet peptides and because somatostatin receptors have been identified in the exocrine pancreas that possibility will be considered first.
Using iodinated analogs of SS-14 and SS-28, high affinity binding sites with the characteristics of receptors have been identified in isolated pancreatic acini, pancreatic membranes and purified plasma membranes (71, 259, 298, 339). Covalent crosslinking studies showed that the binding protein was a 90 kDa glycoprotein (259). Saturable binding sites were also demonstrated in the perfused rat pancreas with autoradiography showing uptake by islets and acinar cells with the acini showing the highest density (89). Molecular cloning has identified 6 different SS receptors, SSRT1- SSRT5 with SSRT2 having two subtypes (249). All of these are 7 transmembrane proteins that are G protein coupled whose action is mediated by Gi/Go (164). SSR2 and SSR5 appear to be the main SS receptor isoforms in acinar and islet cells although further work is needed (26, 124).
Studies directed at actions of somatostatin on pancreatic exocrine cells have primarily been carried out using isolated pancreatic acini which have been separated from islets. SS-14, SS-28 and synthetic analogs have consistently been shown to inhibit cAMP formation through a pertussis toxin sensitive mechanism and in most cases to inhibit amylase secretion stimulated by VIP or secretin which act through cAMP (72, 127, 196, 260, 281). However, most studies reported no inhibition by somatostatin of amylase release stimulated by CCK or cholinergic analogues (72, 127, 214, 280). Rather SS inhibited the potentiating effect of secretin or VIP on CCK stimulation (196, 224). This effect whose size is species-dependent may explain a portion of SS inhibition in vivo but it does not appear to be a major effect. Moreover, it is not clear if it involves islet SS or systemic SS.
The major in vitro technique that allows study of islet SS on the exocrine pancreas is the isolated perfused pancreas where secretion of both islet hormones and exocrine secretion can be measured. Insulin in the perfused pancreas potentiates CCK stimulated exocrine secretion and somatostatin has been established to inhibit both exocrine secretion and insulin secretion (70, 89). In the perfused rat pancreas, high glucose is known to induce secretion of both insulin and SS, and a SS antagonist enhanced amylase secretion induced by glucose plus CCK (238). Depleting SS with cysteamine pretreatment also enhanced CCK stimulated secretion which could be inhibited with exogenous SS. Interestingly, endogenous SS did not modify exocrine secretion stimulated by CCK alone (213, 238). In the perfused pancreas SS also appears to inhibit duct function as fluid secretion stimulated by secretin was potentiated by insulin and inhibited by somatostatin in a manner dependent on Na+- K+ ATPase function (109).
The other proposed locus of somatostatin action to inhibit the exocrine pancreas is at the level of efferent neural control which is initiated in the dorsal motor nucleus of the vagus (DMV) and travels through the vagus nerve to reach pancreatic parenchymal cells. Studies suggesting inhibition at a central vagal site include the finding in rats that somatostatin inhibits the action of 2-deoxyglucose, a known central vagal stimulator (173) and that microinjection of somatostatin into the DMV inhibits pancreatic secretion evoked by CCK or 2DG (174). However, in dogs and rats, other investigators have reported that somatostatin still blocked CCK stimulated secretion after complete pancreatic denervation (29, 269). These and other authors concluded that the inhibitory action of somatostatin is not dependent on the extrinsic nervous system but that the intrinsic nervous system could be involved (214).
C. Pancreatic Polypeptide
Pancreatic polypeptide (PP) was discovered as a contaminant in the purification of insulin in 1975 (144). It contains 36 amino acids and is part of a family of peptides that includes peptide YY (PYY) and neuropeptide Y (NPY) and has about 50% homology to these other peptides (301). The biologically active part of the molecule is in the carboxyl terminal and this part of the molecule extends out from the main globular portion (95). PP is immunogenic and immunohistochemistry has localized it in the islets to a specific cell type originally known as F cells, and now referred to as PP cells (77). These cells are most abundant in the original ventral lobe of the pancreas and some are present outside the islet in the duct epithelium. Small amounts of PP are found in other tissues including the brain (122).
In humans fasting pancreatic polypeptide plasma levels are 10-30 pM, they increase rapidly after feeding and remain elevated for 4-5 hours. The vagal nerve is the main stimulator of PP secretion and secretion can be blocked with atropine. Electrical stimulation of the vagus, sham feeding, 2-deoxyglucose and insulin-induced hypoglycemia all stimulate PP secretion in a vagal dependent manner (270, 327). The half-life of PP in plasma is about 6 minutes. Reported actions of PP include effects on the GI tract, metabolism, most notably reversal of hepatic insulin resistance, and as a satiety factor (327). The latter is based in part on Prader-Willi syndrome where loss of PP secretion is associated with obesity (342). The actions of PP are mediated by specific receptors that belong to a family of receptors that bind NPY, PYY and PP (200). The various receptors are denoted by a capital Y with a numerical subscript. The Y4 receptor has specificity for PP with a high affinity and a hundred-fold lower affinity for PYY.
Purified bovine PP was shown by Lin et al in 1977 to reduce exocrine pancreatic secretion in dogs with pancreatic fistulas (179). This was confirmed by Taylor et al who showed that inhibition of exocrine secretion occurred at doses of porcine PP that raised plasma levels less than seen after a meat meal (302). Similar actions in dogs have been reported by others (16, 39, 185). Shiratori et al. showed a similar effect of human synthetic PP to inhibit canine pancreatic secretion and also showed that immunoneutralization of endogenous PP enhanced both interdigestive and postprandial secretion (274). Similar effects of exogenous PP have been reported in humans (3, 100) and in rats (186, 245).
Although PP acts to inhibit pancreatic secretion in vivo, this effect appears to be indirect as exogenous PP had no effect on amylase release from isolated rat or mouse pancreatic acini (92, 186), the perfused cat pancreas (143), or incubated uncinate pancreas of young rats or pancreatic fragments (143, 186). Binding studies with 125I-PP also failed to reveal high affinity binding sites on rat pancreatic acini. Although this lack of in vitro effects is generally accepted (301), there are several differing reports all using isolated rat pancreatic acini showing a small amount of inhibition (133), stimulation by high concentrations of human PP (59), and inhibition of carbachol but not CCK stimulation by bovine PP (236). Some of these effects could possibly have been due to contaminants in purified PP.
More recent studies have focused on a neural locus for the action of PP to inhibit pancreatic exocrine secretion. Most studies indicate a site of action in the brain stem. Receptors for PP are present in the area postrema, the nuceus tractus solitarius (NTS) and the dorsal motor nucleus of the vagus (320, 321) and intravenous PP inhibits pancreatic enzyme secretion in the rat stimulated with 2-deoxyglucose which acts centrally (245). More definitively, PP microinjected into the DMV in a site specific manner inhibited pancreatic secretion (231). PP directly spritzed on individual DMV neurons revealed a subset where PP reduced postsynaptic currents (30). This finding suggest that PP in the circulation gains access to the brain through the area postrema and reaches the adjacent DMV where it inhibits vagal excitatory output to the pancreas (217). As an alternative neural site for PP inhibition, Jung et al presented data that rat PP inhibited potassium stimulated amylase release and the presynaptic release of acetylcholine in rat pancreatic slices (136). They suggested that PP acts on postganglionic cholinergic neurons to prevent acetylcholine release. The function of inhibition of pancreatic secretion by PP is unclear although several authors have suggested it is to prevent overstimulation of the pancreas.
D. Adrenomedulin
Adrenomedulin (AM) is a 52 amino acid peptide that was isolated from a human pheochromocytoma and has slight primary homology but stronger tertiary resemblance to calcitonin gene related peptide (CGRP). When injected intravenously, AM causes a potent and long lasting hypotensive effect in anesthetized rats (145, 146). AM is present in various organs but most abundantly in the adrenal medulla. It is present diffusely in endocrine cells of the pancreatic islet but most abundantly colocalizes with PP cells where it is present in secretory granules (195). These authors also showed that it is a strong inhibitor of insulin secretion both in vivo and with isolated islets. The AM receptor is made up of a previously orphan receptor, the calcitonin receptor-like receptor (CTL) combined with a receptor-activity-modifying protein (RAMP) (211). There are two AM receptors, AM1 which includes CTLR and RAMP2 and AM2 which is made up of CTLR and RAMP3; the CGRP receptor is CTLR plus RAMP1 (110). In most cells that respond to AM, there is an increase in cAMP (121). In aortic endothelial cells, AM stimulates two signal transduction pathways that increase cAMP and mobilize Ca2+ (273).
Because AM released from PP cells might affect the exocrine pancreas via local blood flow, Tsuchida et al studied the effect of AM on pancreatic acini (308). Radio-iodinated AM was shown to bind to pancreatic acini in a manner inhibited by picomolar concentrations of AM but not by CGRP. AM at concentrations of 1 pM to 1 nM inhibited CCK-stimulated amylase secretion. AM did not affect CCK binding to acini. In contrast to some other cell types, AM did not affect intracellular cAMP or the rise in Ca2+ induced by CCK. Rather, the effect of AM was blocked by pertussis toxin (PTX) suggesting the participation of a PTX sensitive G protein in the action of AM. Additional work on this effect of AM would seem warranted. More recent work on AM in the pancreas has focused on AM produced by pancreatic cancer cells as a mediator of diabetes that often associates with pancreatic cancer, possibly by inhibiting insulin secretion (256).
E. Amylin
Amylin or islet amyloid polypeptide (IAP) was isolated from amyloid rich pancreatic extracts of type 2 diabetic pancreas and shown to be a 37 amino acid peptide that can form oligomers, fibrils and amyloid deposits (44). The amyloid deposits are injurious to the pancreas and are also seen in the brain where they resemble the plaques of Alzheimer’s disease (81). Amyloid is a term for protein aggregation state in which the proteins form a β-sheet. The amyloid deposits are injurious to the pancreas and are also seen in the brain of type 2 diabetics (34). Although amylin is not homologous to the Alzheimer Aβ protein, both can interact with the Amylin receptor which is widely distributed in the brain.
Amylin is produced by islet beta cells, has been localized to the secretory granules (187) and is secreted along with insulin at a ratio of 1:10 to 1:100 (34). Amylin in vivo can inhibit glucagon secretion that occurs at low glucose concentrations and inhibits glucagon secretion from isolated mouse islets (5). The amylin receptor was identified as a heterodimer of a calcitonin seven transmembrane receptor and a single transmembrane receptor activity modifying protein or RAMP (212). These receptors are widely distributed in the brain. Amylin receptor antagonist increased glucagon secretion (90). The acute effects on insulin secretion are more complex as amylin at low concentrations enhanced insulin release while high concentrations inhibited insulin release from mouse islets (5).
A few reports have evaluated the effect of amylin on the exocrine pancreas. In one report amylin had a small effect to increase basal pancreatic secretion in conscious rats (84). In a study in anesthetized rats, Young et al found no effect on basal secretion but a dose-dependent inhibition of CCK stimulated fluid, amylase and lipase secretion up to 67% (337). This effect was seen at low concentrations of amylin (ED50 was 0.11 µg) comparable to effects reported on gastric emptying. However, multiple studies have shown no effect of amylin on secretion by isolated pancreatic acini or AR42J cells (75, 142, 337). Thus, there does not appear to be a direct effect of amylin on pancreatic exocrine cells and the in vivo effects may be on the CNS. There is also no information available on whether amylin receptors exist in the exocrine pancreas.
F. Pancreastatin
Pancreastatin (PST) was first isolated from pig pancreas as a result of screening for peptides with a C-terminal amide structure and shown to inhibit stimulated insulin release (299). While porcine PST is a 49 amino acid peptide, the biological activity resides in the carboxyl half and requires the C-terminal glycine-amide. PST is believed to be derived from chromogranin A as the result of further processing by prohormone convertase 1. The 52 amino acid sequence predicted by the gene structure of human chromogranin A is homologous to porcine PST and when synthesized the full length peptide and its 29 amino acid carboxy-terminal had similar activity to porcine PST to inhibit insulin release (85). Subsequent studies confirmed the inhibition of insulin secretion in vivo, in the perfused pancreas and in isolated pancreatic islets. For reviews see (267, 315). By immunocytochemistry, pancreastatin was shown to be present in the pituitary, adrenal glands, pancreas and throughout the gastrointestinal tract in cells known to contain chromogranin. In the pancreas, PST colocalizes with all four major endocrine cell types and elsewhere is found in neuroendocrine cells (28, 267). Pancreastatin can be measured in plasma by RIA and may serve as a predictive marker for neuroendocrine tumors where PST is elevated (223, 254).
Along with the inhibition of insulin secretion, multiple natural and synthetic forms of PST (human, porcine, bovine, rat) have been shown to inhibit pancreatic digestive enzyme secretion stimulated by CCK in vivo in rats (85, 86, 119) and dogs (55). PST also inhibited pancreatic exocrine secretion stimulated by 2-deoxy-D-Glucose, a central vagal activator (205). By contrast to CCK, pancreatic exocrine secretion stimulated by the cholinergic analog bethanechol in vivo was not blocked by PST. Studies of isolated rat pancreatic acini showed no inhibitory action of PST on CCK stimulation (55, 86, 119, 205, 206) although there is one report of inhibition of CCK stimulation in guinea pig acini (128). Because PST inhibited exocrine pancreatic secretion in vivo but not on isolated cells, the effect of PST is presumed to be indirect.
One possible site of action is on pancreatic blood flow as shown in anesthetized rats using the hydrogen clearance method (201). These investigators found that caerulein enhanced pancreatic blood flow and this increase was dose dependently inhibited by PST at 100 to 500 pmol/kg per h. However, another study in anesthetized dogs showed no effect of PST at similar concentrations using the laser Doppler flowmeter method although protein and amylase secretion were inhibited (55). The other possible site is on the neural stimulatory pathway. Herzig et al showed that when pancreatic lobules which contained neural elements were incubated in vitro, high K+ concentrations stimulated the release of acetylcholine and amylase secretion; both of which were partially inhibited by PST (119). Further studies are necessary to establish pancreastatin as a regulatory peptide and to follow up these potential mechanisms of action. It is also not clear whether PST from a pancreatic or systemic source plays a physiological role in the regulation of exocrine pancreatic function. Absent at present is any information about a PST receptor molecule or PST sensitive ion channel. Moreover, it is not clear what is a physiological concentration of PST. Finally, there is little new information carried out in the last 20 years beyond using PST levels as a tumor marker.
G. Galanin
Galaninin is a 29 amino acid peptide isolated from porcine intestine in 1983 and named for its amino terminal glycine and carboxyl terminal alanine (300). It is considered to be a neuropeptide and is found throughout the body with the highest concentrations in the brain, spinal cord and enteric nervous system (11). Galanin is often co-expressed with other peptides or neurotansmitters including norepinephrine, serotonin, GABA and acetylcholine. Galanin acts via 3 major G protein coupled receptors, GalR1, GalR2 and GalR3 (161). In the pancreas, galanin has been localized mainly to nerves present in both the exocrine and endocrine components but also in some studies to islet cells where colocalization with insulin has been observed (9, 62, 180). In dogs, rodents and humans galanin suppresses insulin release both in vivo and in vitro (161).
A number of studies have evaluated the effect of galanin on isolated rodent acini and the results are presented in the review by Barreto (11). Most show no effect or inhibition. In a study of the isolated, perfused rat pancreas, galanin at low concentrations enhanced both insulin secretion and CCK stimulated amylase secretion (253). On the other hand, using isolated mouse pancreas lobules, galanin had no effect on carbachol stimulation but inhibited CCK stimulated release suggesting an effect on cholinergic transmitter release (13). Galanin also inhibited acetylcholine release stimulated by veratridine in rat pancreatic lobules (118). Overall, these studies suggest galanin is more likely to affect the neural control of exocrine secretion rather than directly affecting acinar cells. Galanin could also affect amylase secretion by blocking somatostatin release (11).
V. References
- Adler G. Effect of glucagon on the secretory process in the rat exocrine pancreas. Cell Tissue Res 182: 193-204, 1977. PMID: 902302.
- Adler G, and Kern HF. Regulation of exocrine pancreatic secretory process by insulin in vivo. Horm Metab Res 7: 290-296, 1975. PMID: 807510.
- Adrian TE, Besterman HS, Mallinson CN, Greenberg GR, and Bloom SR. Inhibition of secretin stimulated pancreatic secretion by pancreatic polypeptide. Gut 20: 37-40, 1979. PMID: 761835.
- Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W, Eckhaus M, Hoffman V, Cui Y, Xiao B, Worley PF, and Muallem S. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab 25: 635-646, 2017. PMID: 28273482.
- Akesson B, Panagiotidis G, Westermark P, and Lundquist I. Islet amyloid polypeptide inhibits glucagon release and exerts a dual action on insulin release from isolated islets. Regul Pept 111: 55-60, 2003. PMID: 12609749.
- Albinus M, Blair EL, Case RM, Coy DH, Gomez-Pan A, Hirst BH, Reed JD, Schally AV, Shaw B, Smith PA, and Smy JR. Comparison of the effect of somatostatin on gastrointestinal function in the conscious and anaesthetized cat and on the isolated cat pancreas. J Physiol 269: 77-91, 1977. PMID: 330838.
- Aughsteen AA, and Kataoka K. Quantitative Radioautographic Study on 3H-Leucine Uptake of Peri- and Tele-Insular Acinar Cells of the Pancreas in Normal and Streptozotocin-Diabetic Mice. Acta Histochem Cytochem 27: 67-74, 1994. DOI: 10.1267/ahc.27.67.
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, and Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113-118, 2000. PMID: 11248802.
- Baltazar ET, Kitamura N, Hondo E, Narreto EC, and Yamada J. Galanin-like immunoreactive endocrine cells in bovine pancreas. J Anat 196 ( Pt 2): 285-291, 2000. PMID: 10739025.
- Bandisode MS, and Singh M. Amylase secretion from isolated pure acinar cells. Biochem Biophys Res Commun 129: 63-69, 1985. PMID: 2408620.
- Barreto SG. Galanin. Pancreapedia: Exocrine Pancreas Knowledge Base, 2015. DOI: 10.3998/panc.2015.21
- Barreto SG, Carati CJ, Toouli J, and Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299: G10-22, 2010. PMID: 20395539.
- Barreto SG, Woods CM, Carati CJ, Schloithe AC, Jaya SR, Toouli J, and Saccone GT. Galanin inhibits caerulein-stimulated pancreatic amylase secretion via cholinergic nerves and insulin. Am J Physiol Gastrointest Liver Physiol297: G333-339, 2009. PMID: 19497960.
- Bartrons R, Simon-Molas H, Rodriguez-Garcia A, Castano E, Navarro-Sabate A, Manzano A, and Martinez-Outschoorn UE. Fructose 2,6-Bisphosphate in Cancer Cell Metabolism. Front Oncol 8: 331, 2018. PMID: 30234009.
- Bazin R, and Lavau M. Diet composition and insulin effect on amylase to lipase ratio in pancreas of diabetic rats. Digestion 19: 386-391, 1979. PMID: 94021.
- Beglinger C, Taylor IL, Grossman MI, and Solomon TE. Pancreatic polypeptide inhibits exocrine pancreatic responses to six stimulants. Am J Physiol Gastrointest Liver Physiol 246: G286-291, 1984. PMID: 6142655.
- Ben Abdeljlil A, Palla JC, and Desnuelle P. Effect of Insulin on Pancreatic Amylase and Chymotrypsinogen. Biochem Biophys Res Commun 18: 71-75, 1965. PMID: 14265760.
- Berg T, Johansen L, and Brekke IB. Insulin potentiates cholecystokinin (CCK)-induced secretion of pancreatic kallikrein. Acta Physiol Scand 123: 89-95, 1985. PMID: 2578723.
- Bergeron JJ, Rachubinski R, Searle N, Sikstrom R, Borts D, Bastian P, and Posner BI. Radioautographic visualization of in vivo insulin binding to the exocrine pancreas. Endocrinology 107: 1069-1080, 1980. PMID: 6997018.
- Berna MJ, Tapia JA, Sancho V, Thill M, Pace A, Hoffmann KM, Gonzalez-Fernandez L, and Jensen RT. Gastrointestinal growth factors and hormones have divergent effects on Akt activation. Cell Signal 21: 622-638, 2009. PMID: 19166928.
- Berry SM, and Fink AS. Insulin inhibits secretin-stimulated pancreatic bicarbonate output by a dose-dependent neurally mediated mechanism. Am J Physiol Gastrointest Liver Physiol 270: G163-170, 1996. PMID: 8772514.
- Biedzinski TM, Bataille D, Devaux MA, and Sarles H. The effect of oxyntomodulin (glucagon-37) and glucagon on exocrine pancreatic secretion in the conscious rat. Peptides 8: 967-972, 1987. PMID: 3441447.
- Boden G, Sivitz MC, Owen OE, Essa-Koumar N, and Landor JH. Somatostatin suppresses secretin and pancreatic exocrine secretion. Science 190: 163-165, 1975. PMID: 1166308.
- Bonner-Weir S, and Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31: 883-889, 1982. PMID: 6759221.
- Bozadjieva N, Williams JA, and Bernal-Mizrachi E. Glucagon. Pancreapedia: Exocrine Pancreas Knowledge base, 2013. DOI: 10.3998/panc.2013.23.
- Braun M. The somatostatin receptor in human pancreatic beta-cells. Vitam Horm 95: 165-193, 2014. PMID: 24559918.
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, and Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77-79, 1973. PMID: 4682131.
- Bretherton-Watt D, Ghatei MA, Bishop AE, Facer P, Fahey M, Hedges M, Williams G, Valentino KL, Tatemoto K, Roth K, Polak JM, and Bloom SR. Pancreastatin distribution and plasma levels in the pig. Peptides 9: 1005-1014, 1988. PMID: 3244555.
- Brodish RJ, Kuvshinoff BW, McFadden DW, and Fink AS. Somatostatin inhibits cholecystokinin-induced pancreatic protein secretion via cholinergic pathways. Pancreas 10: 401-406, 1995. PMID: 7792297.
- Browning KN, Coleman FH, and Travagli RA. Effects of pancreatic polypeptide on pancreas-projecting rat dorsal motor nucleus of the vagus neurons. Am J Physiol Gastrointest Liver Physiol 289: G209-219, 2005. PMID: 15817809.
- Brunicardi FC, Stagner J, Bonner-Weir S, Wayland H, Kleinman R, Livingston E, Guth P, Menger M, McCuskey R, Intaglietta M, Charles A, Ashley S, Cheung A, Ipp E, Gilman S, Howard T, and Passaro E, Jr. Microcirculation of the islets of Langerhans. Long Beach Veterans Administration Regional Medical Education Center Symposium. Diabetes 45: 385-392, 1996. PMID: 8603757.
- Bruzzone R, Trimble ER, Gjinovci A, and Renold AE. Glucose-insulin interactions on exocrine secretion from the perfused rat pancreas. Gastroenterology 87: 1305-1312, 1984. PMID: 6208076.
- Burnham DB, and Williams JA. Effects of carbachol, cholecystokinin, and insulin on protein phosphorylation in isolated pancreatic acini. J Biol Chem 257: 10523-10528, 1982. PMID: 7050109.
- Caillon L, Hoffmann AR, Botz A, and Khemtemourian L. Molecular Structure, Membrane Interactions, and Toxicity of the Islet Amyloid Polypeptide in Type 2 Diabetes Mellitus. J Diabetes Res 2016: 5639875, 2016. PMID: 26636105.
- Cavalot F, Bonomo K, Fiora E, Bacillo E, Salacone P, Chirio M, Gaia E, and Trovati M. Does pancreatic elastase-1 in stools predict steatorrhea in type 1 diabetes? Diabetes Care 29: 719-721, 2006. PMID: 16505538.
- Cecil RL. A Study of the Pathological Anatomy of the Pancreas in Ninety Cases of Diabetes Mellitus. J Exp Med 11: 266-290, 1909. PMID: 19867248.
- Cen ME, Wang F, Su Y, Zhang WJ, Sun B, and Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis 23: 377-387, 2018. PMID: 29926313.
- Chakraborty PP, and Chowdhury S. A Look Inside the Pancreas: The "Endocrine-Exocrine Cross-talk". Endocrinol Metab Synd 4: 1-4, 2015. DOI: 10.4172/ 2161.1017.1000160.
- Chance RE, Cieszkowski M, Jaworek J, Konturek SJ, Swierczek J, and Tasler J. Effect of pancreatic polypeptide and its C-terminal hexapeptide on meal and secretin induced pancreatic secretion in dogs. J Physiol 314: 1-9, 1981. PMID: 7310683.
- Chandrasekar B, and Korc M. Alteration of cholecystokinin-mediated phosphatidylinositol hydrolysis in pancreatic acini from insulin-deficient rats. Evidence for defective G protein activation. Diabetes 40: 1282-1291, 1991. PMID: 1936591.
- Chariot J, Roze C, Vaille C, and Debray C. Effects of somatostatin on the external secretion of the pancreas of the rat. Gastroenterology 75: 832-837, 1978. PMID: 700325.
- Clain JE, Barbezat GO, Waterworth MM, and Bank S. Glucagon inhibition of secretin and combined secretin and cholecystokinin stimulated pancreatic exocrine secretion in health and disease. Digestion 17: 11-17, 1978. PMID: 627317.
- Closa D, Motoo Y, and Iovanna JL. Pancreatitis-associated protein: from a lectin to an anti-inflammatory cytokine. World J Gastroenterol 13: 170-174, 2007. PMID: 17226896.
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, and Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A 84: 8628-8632, 1987. PMID: 3317417.
- Couture Y, Dunnigan J, and Morisset. Stimulation of pancreatic amylase secretion and protein synthesis by insulin. Scand J Gastroenterol 7: 257-263, 1972. PMID: 5034533.
- Cruz J, Posner BI, and Bergeron JJ. Receptor-mediated endocytosis of [125I]insulin into pancreatic acinar cells in vivo. Endocrinology 115: 1996-2008, 1984. PMID: 6386447.
- Danielsson A. Effects of glucose, insulin and glucagon on amylase secretion from incubated mouse pancreas. Pflugers Arch 348: 333-342, 1974. PMID: 4857978.
- Danielsson A, and Sehlin J. Transport and oxidation of amino acids and glucose in the isolated exocrine mouse pancreas: effects of insulin and pancreozymin. Acta Physiol Scand 91: 557-565, 1974. PMID: 4432765.
- De Reggi M, and Gharib B. Protein-X, Pancreatic Stone-, Pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr Protein Pept Sci 2: 19-42, 2001. PMID: 12369899.
- Dembinski A, Warzecha Z, Ceranowicz P, Cieszkowski J, Pawlik WW, Tomaszewska R, Kusnierz-Cabala B, Naskalski JW, Kuwahara A, and Kato I. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res 16: 348-356, 2006. PMID: 17084100.
- Deprez J, Vertommen D, Alessi DR, Hue L, and Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem 272: 17269-17275, 1997. PMID: 9211863.
- DiMagno EP, Go VL, and Summerskill HJ. Intraluminal and postabsorptive effects of amino acids on pancreatic enzyme secretion. J Lab Clin Med 82: 241-248, 1973. PMID: 4721379.
- Dodi G, Militello C, Pedrazzoli S, Zannini G, and Lise M. Exocrine pancreatic function in diabetic rats treated with intraportal islet transplantation. Eur Surg Res 16: 9-14, 1984. PMID: 6321193.
- Doi R, Hosotani R, Inoue K, Kogire M, Sumi S, Fujii N, Yajima H, Rayford PL, and Tobe T. Effect of synthetic human cholecystokinin-33 on pancreatic blood flow in dogs. Pancreas 5: 615-620, 1990. PMID: 2235971.
- Doi R, Inoue K, Hosotani R, Higashide S, Takaori K, Funakoshi S, Yajima H, Rayford PL, and Tobe T. Effects of synthetic human pancreastatin on pancreatic secretion and blood flow in rats and dogs. Peptides 12: 499-502, 1991. PMID: 1717953.
- Dollinger HC, Raptis S, and Pfeiffer EF. Effects of somatostatin on exocrine and endocrine pancreatic function stimulated by intestinal hormones in man. Horm Metab Res 8: 74-78, 1976. PMID: 765255.
- Dranginis A, Morley M, Nesbitt M, Rosenblum BB, and Meisler MH. Independent regulation of nonallelic pancreatic amylase genes in diabetic mice. J Biol Chem 259: 12216-12219, 1984. PMID: 6207174.
- Duan RD, and Erlanson-Albertsson C. Pancreatic lipase and colipase activity increase in pancreatic acinar tissue of diabetic rats. Pancreas 4: 329-334, 1989. PMID: 2471969.
- Duan RD, and Erlanson-Albertsson C. Stimulatory effects of human pancreatic polypeptide on rat pancreatic acini. Regul Pept 12: 215-222, 1985. PMID: 3878538.
- Duan RD, Poensgen J, Wicker C, Westrom B, and Erlanson-Albertsson C. Increase in pancreatic lipase and trypsin activity and their mRNA levels in streptozotocin-induced diabetic rats. Dig Dis Sci 34: 1243-1248, 1989. PMID: 2473868.
- Duan RD, Wicker C, and Erlanson-Albertsson C. Effect of insulin administration on contents, secretion, and synthesis of pancreatic lipase and colipase in rats. Pancreas 6: 595-602, 1991. PMID: 1719525.
- Dunning BE, Ahren B, Veith RC, Bottcher G, Sundler F, and Taborsky GJ, Jr. Galanin: a novel pancreatic neuropeptide. Am J Physiol Endocrinol Metab 251: E127-133, 1986. PMID: 2425633.
- Dunphy JL, Taylor RG, and Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 141: 179-186, 1998. PMID: 9723898.
- Dusetti NJ, Frigerio JM, Keim V, Dagorn JC, and Iovanna JL. Structural organization of the gene encoding the rat pancreatitis-associated protein. Analysis of its evolutionary history reveals an ancient divergence from the other carbohydrate-recognition domain-containing genes. J Biol Chem 268: 14470-14475, 1993. PMID: 8314803.
- Dyck WP, Rudick J, Hoexter B, and Janowitz HD. Influence of glucagon on pancreatic exocrine secretion. Gastroenterology 56: 531-537, 1969. PMID: 5766909.
- Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 320: 1060-1068, 1989. PMID: 2648155.
- Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, and Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 85: 217-224, 2010. PMID: 20176928.
- El-Gohary Y, and Gittes G. Structure of Islets and Vascular Relationship to the Exocrine Pancreas. Pancreapedia: Exocrine Pancreas Knowledge Base, 2017. DOI: 10.3998/panc.2017.10.
- Emoto T, Miyata M, Izukura M, Yumiba T, Mizutani S, Sakamoto T, and Matsuda H. Simultaneous observation of endocrine and exocrine functions of the pancreas responding to somatostatin in man. Regul Pept 68: 1-8, 1997. PMID: 9094748.
- Ensinck JW, Laschansky EC, Vogel RE, and D'Alessio DA. Effect of somatostatin-28 on dynamics of insulin secretion in perfused rat pancreas. Diabetes 40: 1163-1169, 1991. PMID: 1682197.
- Esteve JP, Susini C, Vaysse N, Antoniotti H, Wunsch E, Berthon G, and Ribet A. Binding of somatostatin to pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 247: G62-69, 1984. PMID: 6146265.
- Esteve JP, Vaysse N, Susini C, Kunsch JM, Fourmy D, Pradayrol L, Wunsch E, Moroder L, and Ribet A. Bimodal regulation of pancreatic exocrine function in vitro by somatostatin-28. Am J Physiol Gastrointest Liver Physiol 245: G208-216, 1983. PMID: 6192725.
- Ewald N, and Hardt PD. Alterations in Exocrine Pancreatic Function in Diabetes Mellitus. Pancreapedia: Exocrine Pancreas Knowledge Base, 2015. DOI: 10.3998/panc/2015.7
- Ewald N, Raspe A, Kaufmann C, Bretzel RG, Kloer HU, and Hardt PD. Determinants of Exocrine Pancreatic Function as Measured by Fecal Elastase-1 Concentrations (FEC) in Patients with Diabetes mellitus. Eur J Med Res 14: 118-122, 2009. PMID: 19380282.
- Fehmann HC, Weber V, Goke R, Goke B, Eissele R, and Arnold R. Islet amyloid polypeptide (IAPP;amylin) influences the endocrine but not the exocrine rat pancreas. Biochem Biophys Res Commun 167: 1102-1108, 1990. PMID: 1690993.
- Ferrer R, Medrano J, Diego M, Calpena R, Graells L, Molto M, Perez T, Perez F, and Salido G. Effect of exogenous insulin and glucagon on exocrine pancreatic secretion in rats in vivo. Int J Pancreatol 28: 67-75, 2000. PMID: 11185712.
- Fiocca R, Sessa F, Tenti P, Usellini L, Capella C, O'Hare MM, and Solcia E. Pancreatic polypeptide (PP) cells in the PP-rich lobe of the human pancreas are identified ultrastructurally and immunocytochemically as F cells. Histochemistry 77: 511-523, 1983. PMID: 6345484.
- Foa PP, Santamaria L, Weinstein HR, Berger S, and Smith JA. Secretion of the hyperglycemic-glycogenolytic factor in normal dogs. Am J Physiol 171: 32-36, 1952. PMID: 12985958.
- Folsch UR, Lankisch PG, and Creutzfeldt W. Effect of somatostatin on basal and stimulated pancreatic secretion in the rat. Digestion 17: 194-203, 1978. PMID: 640271.
- Frier BM, Saunders JH, Wormsley KG, and Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut 17: 685-691, 1976. PMID: 976808.
- Fu W, Patel A, and Jhamandas JH. Amylin receptor: a common pathophysiological target in Alzheimer's disease and diabetes mellitus. Front Aging Neurosci 5: 42, 2013. PMID: 23966942.
- Fujita T. Insulo-acinar portal system in the horse pancreas. Arch Histol Jpn 35: 161-171, 1973. PMID: 4573520.
- Fujita T, and Murakami T. Microcirculation of monkey pancreas with special reference to the insulo-acinar portal system. A scanning electron microscope study of vascular casts. Arch Histol Jpn 35: 255-263, 1973. PMID: 4198293.
- Funakoshi A, Miyasaka K, Kitani K, Nakamura J, Funakoshi S, Fukuda H, and Fujii N. Stimulatory effects of islet amyloid polypeptide (amylin) on exocrine pancreas and gastrin release in conscious rats. Regul Pept 38: 135-143, 1992. PMID: 1574608.
- Funakoshi A, Miyasaka K, Nakamura R, Kitani K, Funakoshi S, Tamamura H, Fujii N, and Yajima H. Bioactivity of synthetic human pancreastatin on exocrine pancreas. Biochem Biophys Res Commun 156: 1237-1242, 1988. PMID: 3190702.
- Funakoshi A, Miyasaka K, Nakamura R, Kitani K, and Tatemoto K. Inhibitory effect of pancreastatin on pancreatic exocrine secretion in the conscious rat. Regul Pept 25: 157-166, 1989. PMID: 2474177.
- Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, and Steiner DF. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem 276: 27197-27202, 2001. PMID: 11356850.
- Garcia TS, Rech TH, and Leitao CB. Pancreatic size and fat content in diabetes: A systematic review and meta-analysis of imaging studies. PLoS One 12: e0180911, 2017. PMID: 28742102.
- Garry DJ, Garry MG, Williams JA, Mahoney WC, and Sorenson RL. Effects of islet hormones on amylase secretion and localization of somatostatin binding sites. Am J Physiol Gastrointest Liver Physiol 256: G897-904, 1989. PMID: 2470260.
- Gedulin BR, Jodka CM, Herrmann K, and Young AA. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul Pept 137: 121-127, 2006. PMID: 16914214.
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619-633, 1965. PMID: 5318831.
- Gettys TW, Tanaka I, and Taylor IL. Modulation of pancreatic exocrine function in rodents by treatment with pancreatic polypeptide. Pancreas 7: 705-711, 1992. PMID: 1280366.
- Girman CJ, Kou TD, Cai B, Alexander CM, O'Neill EA, Williams-Herman DE, and Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 12: 766-771, 2010. PMID: 20649628.
- Gironella M, Folch-Puy E, LeGoffic A, Garcia S, Christa L, Smith A, Tebar L, Hunt SP, Bayne R, Smith AJ, Dagorn JC, Closa D, and Iovanna JL. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut 56: 1091-1097, 2007. PMID: 17409121.
- Glover ID, Barlow DJ, Pitts JE, Wood SP, Tickle IJ, Blundell TL, Tatemoto K, Kimmel JR, Wollmer A, Strassburger W, and Zhang Y-S. Conformational studies on the pancreatic polypeptide hormone family. Eur J Biochem 142: 379-385, 1984. PMID: 6745282.
- Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, and Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol 38: 145-149, 2001. PMID: 11827436.
- Goldfine ID, Kriz BM, Wong KY, Hradek G, Jones AL, and Williams JA. Insulin action in pancreatic acini from streptozotocin-treated rats. III. Electron microscope autoradiography of 125I-insulin. Am J Physiol Gastrointest Liver Physiol 240: G69-75, 1981. PMID: 7006419.
- Goldfine ID, and Williams JA. Receptors for insulin and CCK in the acinar pancreas: relationship to hormone action. Int Rev Cytol 85: 1-38, 1983. PMID: 6198304.
- Gonzalez-Perez A, Schlienger RG, and Rodriguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 33: 2580-2585, 2010. PMID: 20833867.
- Greenberg GR, McCloy RF, Adrian TE, Chadwick VS, Baron JH, and Bloom SR. Inhibition of pancreas and gallbladder by pancreatic polypeptide. Lancet 2: 1280-1282, 1978. PMID: 82783.
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, and Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 180: 821-827, 2009. PMID: 19318387.
- Gromada J, Franklin I, and Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84-116, 2007. PMID: 17261637.
- Gyr K, Beglinger C, Kohler E, Trautzl U, Keller U, and Bloom SR. Circulating somatostatin. Physiological regulator of pancreatic function? J Clin Invest 79: 1595-1600, 1987. PMID: 2884233.
- Hansen LH, Abrahamsen N, and Nishimura E. Glucagon receptor mRNA distribution in rat tissues. Peptides 16: 1163-1166, 1995. PMID: 8532603.
- Harada H, Kochi F, Hanafusa E, Kobayashi T, Oka H, and Kimura I. Studies on the effect of glucagon on human pancreatic secretion by analysis of endoscopically obtained pure pancreatic juice. Gastroenterol Jpn 20: 28-36, 1985. PMID: 4018495.
- Hardt PD, Brendel MD, Kloer HU, and Bretzel RG. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care 31 Suppl 2: S165-169, 2008. PMID: 18227480.
- Hardt PD, Hauenschild A, Jaeger C, Teichmann J, Bretzel RG, Kloer HU, and Group SSS. High prevalence of steatorrhea in 101 diabetic patients likely to suffer from exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations: a prospective multicenter study. Dig Dis Sci 48: 1688-1692, 2003. PMID: 14560984.
- Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Jaeger C, Teichmann J, Bretzel RG, Hollenhorst M, Kloer HU, and Group SSS. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology 3: 395-402, 2003. PMID: 14526149.
- Hasegawa H, Okabayashi Y, Koide M, Kido Y, Okutani T, Matsushita K, Otsuki M, and Kasuga M. Effect of islet hormones on secretin-stimulated exocrine secretion in isolated perfused rat pancreas. Dig Dis Sci 38: 1278-1283, 1993. PMID: 8325188.
- Hay DL, Garelja ML, Poyner DR, and Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol 175: 3-17, 2018. PMID: 29059473.
- Hegyi P, Rakonczay Z, Jr., Sari R, Czako L, Farkas N, Gog C, Nemeth J, Lonovics J, and Takacs T. Insulin is necessary for the hypertrophic effect of cholecystokinin-octapeptide following acute necrotizing experimental pancreatitis. World J Gastroenterol 10: 2275-2277, 2004. PMID: 15259081.
- Hegyi P, Rakonczay Z, Jr., Sari R, Gog C, Lonovics J, Takacs T, and Czako L. L-arginine-induced experimental pancreatitis. World J Gastroenterol 10: 2003-2009, 2004. PMID: 15237423.
- Hegyi P, Takacs T, Jarmay K, Nagy I, Czako L, and Lonovics J. Spontaneous and cholecystokinin-octapeptide-promoted regeneration of the pancreas following L-arginine-induced pancreatitis in rat. Int J Pancreatol 22: 193-200, 1997. PMID: 9444550.
- Hegyi P, Takacs T, Tiszlavicz L, Czako L, and Lonovics J. Recovery of exocrine pancreas six months following pancreatitis induction with L-arginine in streptozotocin-diabetic rats. J Physiol Paris 94: 51-55, 2000. PMID: 10761689.
- Henderson JR, and Daniel PM. A comparative study of the portal vessels connecting the endocrine and exocrine pancreas, with a discussion of some functional implications. Q J Exp Physiol 64: 267-275, 1979. PMID: 118478.
- Henderson JR, Daniel PM, and Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 22: 158-167, 1981. PMID: 6111521.
- Henderson JR, and Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol 70: 347-356, 1985. PMID: 3898188.
- Herzig KH, Brunke G, Schon I, Schaffer M, and Folsch UR. Mechanism of galanin's inhibitory action on pancreatic enzyme secretion: modulation of cholinergic transmission--studies in vivo and in vitro. Gut 34: 1616-1621, 1993. PMID: 7694889.
- Herzig KH, Louie DS, Tatemoto K, and Owyang C. Pancreastatin inhibits pancreatic enzyme secretion by presynaptic modulation of acetylcholine release. Am J Physiol Gastrointest Liver Physiol 262: G113-117, 1992. PMID: 1370747.
- Hildebrand P, Ensinck JW, Gyr K, Mossi S, Leuppi J, Eggenberger C, and Beglinger C. Evidence for hormonal inhibition of exocrine pancreatic function by somatostatin 28 in humans. Gastroenterology 103: 240-247, 1992. PMID: 1351858.
- Hinson JP, Kapas S, and Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21: 138-167, 2000. PMID: 10782362.
- Holzer P, Reichmann F, and Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46: 261-274, 2012. PMID: 22979996.
- Horiguchi Y. Interaction of secretin and glucagon on exocrine pancreatic secretion. Gastroenterol Jpn 14: 63-73, 1979. PMID: 446988.
- Hunyady B, Hipkin RW, Schonbrunn A, and Mezey E. Immunohistochemical localization of somatostatin receptor SST2A in the rat pancreas. Endocrinology 138: 2632-2635, 1997. PMID: 9165058.
- Inayat F, Zafar F, Riaz I, Younus F, Baig AS, and Imran Z. Hypertriglyceridemic Pancreatitis: Is Insulin Monotherapy A Feasible Therapeutic Option? Cureus 10: e3461, 2018. PMID: 30564540.
- Investigators COIITSS Study; Annane D, Cariou A, Maxime V, Azoulay E, D'Honneur G, Timsit JF, Cohen Y, Wolf M, Fartoukh M, Adrie C, Santre C, Bollaert PE, Mathonet A, Amathieu R, Tabah A, Clec'h C, Mayaux J, Lejeune J, and Chevret S. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 303: 341-348, 2010. PMID: 20103758.
- Ishiguro H, Hayakawa T, Kondo T, Shibata T, Kitagawa M, Sakai Y, Sobajima H, Nakae Y, and Tanikawa M. The effect of somatostatin analogue octreotide on amylase secretion from mouse pancreatic acini. Digestion 54: 207-212, 1993. PMID: 7694882.
- Ishizuka J, Asada I, Poston GJ, Lluis F, Tatemoto K, Greeley GH, Jr., and Thompson JC. Effect of pancreastatin on pancreatic endocrine and exocrine secretion. Pancreas 4: 277-281, 1989. PMID: 2471966.
- Jansson L. Vasoactive intestinal polypeptide increases whole pancreatic blood flow but does not affect islet blood flow in the rat. Acta Diabetol 31: 103-106, 1994. PMID: 7949220.
- Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Kallskog O, Lau J, Liljeback H, Palm F, Quach M, Sandberg M, Stromberg V, Ullsten S, and Carlsson PO. Pancreatic islet blood flow and its measurement. Ups J Med Sci 121: 81-95, 2016. PMID: 27124642.
- Jansson L, and Hellerstrom C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia 25: 45-50, 1983. PMID: 6350083.
- Jefferson LS. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes 29: 487-496, 1980. PMID: 6991336.
- Joehl RJ, and DeJoseph MR. Pancreatic polypeptide inhibits amylase release by rat pancreatic acini. J Surg Res 40: 310-314, 1986. PMID: 2422440.
- Johnson TM, Rosenberg MP, and Meisler MH. An insulin-responsive element in the pancreatic enhancer of the amylase gene. J Biol Chem 268: 464-468, 1993. PMID: 7678001.
- Juma LM, Singh J, Pallot DJ, Salido GM, and Adeghate E. Interactions of islet hormones with acetylcholine in the isolated rat pancreas. Peptides 18: 1415-1422, 1997. PMID: 9392845.
- Jung G, Louie DS, and Owyang C. Pancreatic polypeptide inhibits pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol Gastrointest Liver Physiol 253: G706-710, 1987. PMID: 2446510.
- Kanno T, and Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol 261: 505-521, 1976. PMID: 978585.
- Kayasseh L, Gyr K, Stalder GA, Rittmann WW, and Girard J. Effect of somatostatin on exocrine pancreatic secretion stimulated by pancreozymin-secretin or by a test meal in the dog. Horm Res 9: 176-184, 1978. PMID: 640582.
- Keim V, Rohr G, Stockert HG, and Haberich FJ. An additional secretory protein in the rat pancreas. Digestion 29: 242-249, 1984. PMID: 6468771.
- Keller SA, Rosenberg MP, Johnson TM, Howard G, and Meisler MH. Regulation of amylase gene expression in diabetic mice is mediated by a cis-acting upstream element close to the pancreas-specific enhancer. Genes Dev 4: 1316-1321, 1990. PMID: 1699843.
- Kemmer TP, Malfertheiner P, Buchler M, Friess H, Meschenmoser L, and Ditschuneit H. Inhibition of human exocrine pancreatic secretion by the long-acting somatostatin analogue octreotide (SMS 201-995). Aliment Pharmacol Ther 6: 41-50, 1992. PMID: 1371938.
- Kikuchi Y, Koizumi M, Shimosegawa T, Kashimura J, Suzuki S, and Toyota T. Islet amyloid polypeptide has no effect on amylase release from rat pancreatic acini stimulated by CCK-8, secretin, carbachol and VIP. Tohoku J Exp Med 165: 41-48, 1991. PMID: 1724710.
- Kim KH, and Case RM. Effects of pancreatic polypeptide on the secretion of enzymes and electrolytes by in vitro preparations of rat and cat pancreas. Yonsei Med J 21: 99-105, 1980. PMID: 6171935.
- Kimmel JR, Hayden LJ, and Pollock HG. Isolation and characterization of a new pancreatic polypeptide hormone. J Biol Chem 250: 9369-9376, 1975. PMID: 1194289.
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, and Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553-560, 1993. PMID: 8387282.
- Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, and Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun 194: 720-725, 1993. PMID: 7688224.
- Kohler E, Beglinger C, Ribes G, Grotzinger U, Loubatieres-Mariani MM, and Gyr K. Effect of circulating somatostatin on exocrine pancreatic secretion in conscious dogs. Pancreas 1: 455-459, 1986. PMID: 2882502.
- Koizumi M, Yoshida Y, Abe N, Shimosegawa T, and Toyota T. Pancreatic diabetes in Japan. Pancreas 16: 385-391, 1998. PMID: 9548683.
- Komabayashi T, Sawada H, Izawa T, and Kogo H. Altered intracellular Ca2+ regulation in pancreatic acinar cells from acute streptozotocin-induced diabetic rats. Eur J Pharmacol 298: 299-306, 1996. PMID: 8846830.
- Konturek SJ, Bilski J, Jaworek J, Tasler J, and Schally AV. Comparison of somatostatin and its highly potent hexa- and octapeptide analogs on exocrine and endocrine pancreatic secretion. Proc Soc Exp Biol Med 187: 241-249, 1988. PMID: 2448802.
- Konturek SJ, Demitrescu T, Radecki T, Thor P, and Pucher A. Effect of glucagon on gastric and pancreatic secretion and peptic ulcer formation in cats. Am J Dig Dis 19: 557-564, 1974. PMID: 4597820.
- Konturek SJ, Tasler J, and Obtulowicz W. Characteristics of inhibition of pancreatic secretion by glucagon. Digestion 10: 138-149, 1974. PMID: 4841910.
- Korc M, Bailey AC, and Williams JA. Regulation of protein synthesis in normal and diabetic rat pancreas by cholecystokinin. Am J Physiol Gastrointest Liver Physiol 241: G116-121, 1981. PMID: 6791509.
- Korc M, Iwamoto Y, Sankaran H, Williams JA, and Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. I. Stimulation of protein synthesis. Am J Physiol Gastrointest Liver Physiol 240: G56-62, 1981. PMID: 6161541.
- Korc M, Owerbach D, Quinto C, and Rutter WJ. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science 213: 351-353, 1981. PMID: 6166044.
- Korc M, Sankaran H, Wong KY, Williams JA, and Goldfine ID. Insulin receptors in isolated mouse pancreatic acini. Biochem Biophys Res Commun 84: 293-299, 1978. PMID: 363126.
- Kramer MF, and Poort C. The effect of insulin on amino acid incorporation into exocrine pancreatic cells of the rat. Horm Metab Res 7: 389-393, 1975. PMID: 1183917.
- Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, Maurer PM, Schultz MJ, van Hooijdonk RT, Kiyoshi M, Mackenzie IM, Annane D, Stow P, Nasraway SA, Holewinski S, Holzinger U, Preiser JC, Vincent JL, and Bellomo R. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 17: R37, 2013. PMID: 23452622.
- Krogh-Madsen R, Moller K, Dela F, Kronborg G, Jauffred S, and Pedersen BK. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol 286: E766-772, 2004. PMID: 14722028.
- Lahaie RG. Translational control of protein synthesis in isolated pancreatic acini: role of CCK8, carbachol, and insulin. Pancreas 1: 403-410, 1986. PMID: 2436215.
- Lang R, Gundlach AL, and Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther 115: 177-207, 2007. PMID: 17604107.
- Lankisch PG, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, and Creutzfeldt W. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion 25: 211-216, 1982. PMID: 6186557.
- Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobecourt E, and Boitard C. Pancreatic exocrine function in patients with diabetes. Diabet Med 29: 1047-1054, 2012. PMID: 22273174.
- Law SF, Manning D, and Reisine T. Identification of the subunits of GTP-binding proteins coupled to somatostatin receptors. J Biol Chem 266: 17885-17897, 1991. PMID: 1655730.
- Lee KY, Krusch D, Zhou L, Song Y, Chang TM, and Chey WY. Effect of endogenous insulin on pancreatic exocrine secretion in perfused dog pancreas. Pancreas 11: 190-195, 1995. PMID: 7479678.
- Lee KY, Lee YL, Kim CD, Chang TM, and Chey WY. Mechanism of action of insulin on pancreatic exocrine secretion in perfused rat pancreas. Am J Physiol Gastrointest Liver Physiol 267: G207-212, 1994. PMID: 7915495.
- Lee KY, Zhou L, Ren XS, Chang TM, and Chey WY. An important role of endogenous insulin on exocrine pancreatic secretion in rats. Am J Physiol Gastrointest Liver Physiol 258: G268-274, 1990. PMID: 1689549.
- Lee PC, Jordan M, Pieper GM, and Roza AM. Normalization of pancreatic exocrine enzymes by islet transplantation in diabetic rats. Biochem Cell Biol 73: 269-273, 1995. PMID: 8829373.
- Lee YL, Kwon HY, Park HS, Lee TH, and Park HJ. The role of insulin in the interaction of secretin and cholecystokinin in exocrine secretion of the isolated perfused rat pancreas. Pancreas 12: 58-63, 1996. PMID: 8927620.
- Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, Wilinska ME, Nodale M, Mangat J, Evans ML, Burnstein R, and Hovorka R. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care 17: R159, 2013. PMID: 23883613.
- Lefebvre PJ. Glucagon and its family revisited. Diabetes Care 18: 715-730, 1995. PMID: 8586014.
- Levy P, Milan C, Pignon JP, Baetz A, and Bernades P. Mortality factors associated with chronic pancreatitis. Unidimensional and multidimensional analysis of a medical-surgical series of 240 patients. Gastroenterology 96: 1165-1172, 1989. PMID: 2925060.
- Li Y, and Owyang C. Somatostatin inhibits pancreatic enzyme secretion at a central vagal site. Am J Physiol Gastrointest Liver Physiol 265: G251-257, 1993. PMID: 8103634.
- Liao Z, Li ZS, Lu Y, and Wang WZ. Microinjection of exogenous somatostatin in the dorsal vagal complex inhibits pancreatic secretion via somatostatin receptor-2 in rats. Am J Physiol 292: G746-752, 2007. PMID: 17138968.
- Lifson N, Kramlinger KG, Mayrand RR, and Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology 79: 466-473, 1980. PMID: 7000613.
- Lifson N, and Lassa CV. Note on the blood supply of the ducts of the rabbit pancreas. Microvasc Res 22: 171-176, 1981. PMID: 7033729.
- Lifson N, Lassa CV, and Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol Endocrinol Metab 249: E43-48, 1985. PMID: 2409813.
- Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim KM, Choi SH, Park KS, Florez JC, and Jang HC. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol 51: 739-748, 2014. PMID: 24671510.
- Lin TM, Evans DC, Chance RE, and Spray GF. Bovine pancreatic peptide: action on gastric and pancreatic secretion in dogs. Am J Physiol 232: E311-315, 1977. PMID: 842664.
- Lindskog S, Ahren B, Dunning BE, and Sundler F. Galanin-immunoreactive nerves in the mouse and rat pancreas. Cell Tissue Res 264: 363-368, 1991. PMID: 1715243.
- Ling Y, Li X, and Gao X. Intensive versus conventional glucose control in critically ill patients: a meta-analysis of randomized controlled trials. Eur J Intern Med 23: 564-574, 2012. PMID: 22863436.
- Lithell H, Vessby B, Walldius G, and Carlson LA. Hypertriglyceridemia--acute pancreatitis--ischemic heart disease. A case study in a pair of monozygotic twins. Acta Med Scand 221: 311-316, 1987. PMID: 3591470.
- Liu YM, Guth PH, Kaneko K, Livingston EH, and Brunicardi FC. Dynamic in vivo observation of rat islet microcirculation. Pancreas 8: 15-21, 1993. PMID: 8419903.
- Lohr M, and Kloppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 30: 757-762, 1987. PMID: 3322901.
- Lonovics J, Guzman S, Devitt PG, Hejtmancik KE, Suddith RL, Rayford PL, and Thompson JC. Action of pancreatic polypeptide on exocrine pancreas and on release of cholecystokinin and secretin. Endocrinology 108: 1925-1930, 1981. PMID: 7215307.
- Louie DS, Williams JA, and Owyang C. Action of pancreatic polypeptide on rat pancreatic secretion: in vivo and in vitro. Am J Physiol Gastrointest Liver Physiol 249: G489-495, 1985. PMID: 2413769.
- Lukinius A, Wilander E, Westermark GT, Engstrom U, and Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 32: 240-244, 1989. PMID: 2668077.
- Macauley M, Percival K, Thelwall PE, Hollingsworth KG, and Taylor R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One 10: e0126825, 2015. PMID: 25950180.
- Maclaren DP, Mohebbi H, Nirmalan M, Keegan MA, Best CT, Perera D, Harvie MN, and Campbell IT. Effect of a 2-h hyperglycemic-hyperinsulinemic glucose clamp to promote glucose storage on endurance exercise performance. Eur J Appl Physiol 111: 2105-2114, 2011. PMID: 21286922.
- Mailliard ME, Stevens BR, and Mann GE. Amino acid transport by small intestinal, hepatic, and pancreatic epithelia. Gastroenterology 108: 888-910, 1995. PMID: 7875494.
- Mankad P, James A, Siriwardena AK, Elliott AC, and Bruce JI. Insulin protects pancreatic acinar cells from cytosolic calcium overload and inhibition of the plasma membrane calcium pump. J Biol Chem 287: 1823-1836, 2012. PMID: 22128146.
- Mann GE, Habara Y, and Peran S. Characteristics of L-glutamine transport in the perfused rat exocrine pancreas: lack of sensitivity to insulin and streptozotocin-induced experimental diabetes. Pancreas 1: 239-245, 1986. PMID: 3106960.
- Mann GE, and Norman PS. Regulatory effects of insulin and experimental diabetes on neutral amino acid transport in the perfused rat exocrine pancreas. Kinetics of unidirectional L-serine influx and efflux at the basolateral plasma membrane. Biochim Biophys Acta 778: 618-622, 1984. PMID: 6439248.
- Maouyo D, and Morisset J. Modulation of pancreatic secretion of individual digestive enzymes in octreotide (SMS 201-995)-infused rats. Pancreas 14: 47-57, 1997. PMID: 8981507.
- Martinez A, Weaver C, Lopez J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, and Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology 137: 2626-2632, 1996. PMID: 8641217.
- Matsushita K, Okabayashi Y, Hasegawa H, Koide M, Kido Y, Okutani T, Sugimoto Y, and Kasuga M. In vitro inhibitory effect of somatostatin on secretin action in exocrine pancreas of rats. Gastroenterology 104: 1146-1152, 1993. PMID: 7681794.
- Matsushita K, Okabayashi Y, Koide M, Hasegawa H, Otsuki M, and Kasuga M. Potentiating effect of insulin on exocrine secretory function in isolated rat pancreatic acini. Gastroenterology 106: 200-206, 1994. PMID: 7506218.
- McCuskey RS, and Chapman TM. Microscopy of the living pancreas in situ. Am J Anat 126: 395-407, 1969. PMID: 5369107.
- Mentula P, Kylanpaa ML, Kemppainen E, and Puolakkainen P. Obesity correlates with early hyperglycemia in patients with acute pancreatitis who developed organ failure. Pancreas 36: e21-25, 2008. PMID: 18192869.
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, and Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50: 143-150, 1998. PMID: 9549761.
- Migita Y, Nakano I, Goto M, Ito T, and Nawata H. Effect of pancreastatin on cerulein-stimulated pancreatic blood flow and exocrine secretion in anaesthetized rats. J Gastroenterol Hepatol 14: 583-587, 1999. PMID: 10385069.
- Miko A, Farkas N, Garami A, Szabo I, Vincze A, Veres G, Bajor J, Alizadeh H, Rakonczay Z, Jr., Vigh E, Marta K, Kiss Z, Hegyi P, and Czako L. Preexisting Diabetes Elevates Risk of Local and Systemic Complications in Acute Pancreatitis: Systematic Review and Meta-analysis. Pancreas 47: 917-923, 2018. PMID: 30113426.
- Miller TA, Tepperman FS, Fang WF, and Jacobson ED. Effect of somatostatin on pancreatic protein secretion induced by cholecystokinin. J Surg Res 26: 488-493, 1979. PMID: 439879.
- Miyake T, Murakami T, and Ohtsuka A. Incomplete vascular casting for a scanning electron microscope study of the microcirculatory patterns in the rat pancreas. Arch Histol Cytol 55: 397-406, 1992. PMID: 1482604.
- Miyasaka K, Funakoshi A, Kitani K, Tamamura H, Funakoshi S, and Fujii N. Inhibitory effect of pancreastatin on pancreatic exocrine secretions. Pancreastatin inhibits central vagal nerve stimulation. Gastroenterology 99: 1751-1756, 1990. PMID: 1977653.
- Miyasaka K, Funakoshi A, Yasunami Y, Nakamura R, Kitani K, Tamamura H, Funakoshi S, and Fujii N. Rat pancreastatin inhibits both pancreatic exocrine and endocrine secretions in rats. Regul Pept 28: 189-198, 1990. PMID: 1693005.
- Mohapatra S, Majumder S, Smyrk TC, Zhang L, Matveyenko A, Kudva YC, and Chari ST. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas 45: 1104-1110, 2016. PMID: 26918874.
- Morisset J. Somatostatin. Pancreapedia: Exocrine Pancreas Knowledge base, 2015. DOI: 10.3998/ 2015.43.
- Mossner J, Logsdon CD, Goldfine ID, and Williams JA. Regulation of pancreatic acinar cell insulin receptors by insulin. Am J Physiol Gastrointest Liver Physiol 247: G155-160, 1984. PMID: 6380310.
- Mossner J, Logsdon CD, Williams JA, and Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar AR42J cells. Diabetes 34: 891-897, 1985. PMID: 2411617.
- Muff R, Born W, and Fischer JA. Adrenomedullin and related peptides: receptors and accessory proteins. Peptides 22: 1765-1772, 2001. PMID: 11754962.
- Muff R, Buhlmann N, Fischer JA, and Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology 140: 2924-2927, 1999. PMID: 10342886.
- Muller MK, Kessel B, Hutt T, Kath R, Layer P, and Goebell H. Effects of somatostatin-14 on gastric and pancreatic responses to hormonal and neural stimulation using an isolated perfused rat stomach and pancreas preparation. Pancreas 3: 303-310, 1988. PMID: 2455292.
- Mulvihill SJ, Bunnett NW, Goto Y, and Debas HT. Somatostatin inhibits pancreatic exocrine secretion via a neural mechanism. Metabolism 39: 143-148, 1990. PMID: 1698248.
- Munoz M, Sweiry JH, and Mann GE. Insulin stimulates cationic amino acid transport activity in the isolated perfused rat pancreas. Exp Physiol 80: 745-753, 1995. PMID: 8546864.
- Murakami T, Fujita T, Taguchi T, Nonaka Y, and Orita K. The blood vascular bed of the human pancreas, with special reference to the insulo-acinar portal system. Scanning electron microscopy of corrosion casts. Arch Histol Cytol 55: 381-395, 1992. PMID: 1482603.
- Mussa BM, and Verberne AJ. The dorsal motor nucleus of the vagus and regulation of pancreatic secretory function. Exp Physiol 98: 25-37, 2013. PMID: 22660814.
- Nakagawa A, Samols E, and Stagner JI. Exocrine interstitial insulin and somatostatin in the perfused dog pancreas. Am J Physiol Gastrointest Liver Physiol 264: G728-734, 1993. PMID: 8097377.
- Nakagawa A, Stagner JI, and Samols E. In situ binding of islet hormones in the isolated perfused rat pancreas: evidence for local high concentrations of islet hormones via the islet-acinar axis. Diabetologia 38: 262-268, 1995. PMID: 7758870.
- Noel RA, Braun DK, Patterson RE, and Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 32: 834-838, 2009. PMID: 19208917.
- Norman PS, Habara Y, and Mann GE. Paradoxical effects of endogenous and exogenous insulin on amino acid transport activity in the isolated rat pancreas: somatostatin-14 inhibits insulin action. Diabetologia 32: 177-184, 1989. PMID: 2568959.
- Nunes AC, Pontes JM, Rosa A, Gomes L, Carvalheiro M, and Freitas D. Screening for pancreatic exocrine insufficiency in patients with diabetes mellitus. Am J Gastroenterol 98: 2672-2675, 2003. PMID: 14687815.
- O'Dorisio TM, Krutzik SR, Woltering EA, Lindholm E, Joseph S, Gandolfi AE, Wang YZ, Boudreaux JP, Vinik AI, Go VL, Howe JR, Halfdanarson T, O'Dorisio MS, and Mamikunian G. Development of a highly sensitive and specific carboxy-terminal human pancreastatin assay to monitor neuroendocrine tumor behavior. Pancreas 39: 611-616, 2010. PMID: 20124939.
- Ohnishi H, Mine T, and Kojima I. Inhibition by somatostatin of amylase secretion induced by calcium and cyclic AMP in rat pancreatic acini. Biochem J 304 ( Pt 2): 531-536, 1994. PMID: 7528010.
- Ohtani O, Ushiki T, Kanazawa H, and Fujita T. Microcirculation of the pancreas in the rat and rabbit with special reference to the insulo-acinar portal system and emissary vein of the islet. Arch Histol Jpn 49: 45-60, 1986. PMID: 3524504.
- Okabayashi Y, Maddux BA, McDonald AR, Logsdon CD, Williams JA, and Goldfine ID. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes 38: 182-187, 1989. PMID: 2644141.
- Okabayashi Y, Moessner J, Logsdon CD, Goldfine ID, and Williams JA. Insulin and other stimulants have nonparallel translational effects on protein synthesis. Diabetes 36: 1054-1060, 1987. PMID: 3301474.
- Okabayashi Y, Otsuki M, Nakamura T, Koide M, Hasegawa H, Okutani T, and Kido Y. Regulatory effect of cholecystokinin on subsequent insulin binding to pancreatic acini. Am J Physiol Endocrinol Metab 258: E562-568, 1990. PMID: 2110419.
- Okabayashi Y, Otsuki M, Ohki A, Nakamura T, Tani S, and Baba S. Secretin-induced exocrine secretion in perfused pancreas isolated from diabetic rats. Diabetes 37: 1173-1180, 1988. PMID: 2457529.
- Okabayashi Y, Otsuki M, Ohki A, Suehiro I, and Baba S. Effect of diabetes mellitus on pancreatic exocrine secretion from isolated perfused pancreas in rats. Dig Dis Sci 33: 711-717, 1988. PMID: 3286155.
- Okumura T, Pappas TN, and Taylor IL. Pancreatic polypeptide microinjection into the dorsal motor nucleus inhibits pancreatic secretion in rats. Gastroenterology 108: 1517-1525, 1995. PMID: 7729645.
- Orelle B, Keim V, Masciotra L, Dagorn JC, and Iovanna JL. Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J Clin Invest 90: 2284-2291, 1992. PMID: 1469087.
- Otsuki M, Goldfine ID, and Williams JA. Diabetes in the rat is associated with a reversible postreceptor defect in cholecystokinin action. Gastroenterology 87: 882-887, 1984. PMID: 6205933.
- Otsuki M, and Williams JA. Direct modulation of pancreatic CCK receptors and enzyme secretion by insulin in isolated pancreatic acini from diabetic rats. Diabetes 32: 241-246, 1983. PMID: 6186556.
- Otsuki M, and Williams JA. Effect of diabetes mellitus on the regulation of enzyme secretion by isolated rat pancreatic acini. J Clin Invest 70: 148-156, 1982. PMID: 6177717.
- Pan GZ, Lu L, Qian JM, and Xue BG. Bovine pancreatic polypeptide as an antagonist of muscarinic cholinergic receptors. Am J Physiol Gastrointest Liver Physiol 252: G384-391, 1987. PMID: 2435168.
- Pandol SJ, Sutliff VE, Jones SW, Charlton CG, O'Donohue TL, Gardner JD, and Jensen RT. Action of natural glucagon on pancreatic acini: due to contamination by previously undescribed secretagogues. Am J Physiol Gastrointest Liver Physiol 245: G703-710, 1983. PMID: 6195929.
- Park HS, Yoon HS, Park YD, Cui ZY, Lee YL, and Park HJ. Endogenous somatostatin inhibits interaction of insulin and cholecystokinin on exocrine secretion of isolated, perfused rat pancreas. Pancreas 24: 373-379, 2002. PMID: 11961490.
- Patel R, Pariente JA, Martinez MA, Salido GM, and Singh J. Effect of insulin on acetylcholine-evoked amylase release and calcium mobilization in streptozotocin-induced diabetic rat pancreatic acinar cells. Ann N Y Acad Sci 1084: 58-70, 2006. PMID: 17151293.
- Patel R, Shervington A, Pariente JA, Martinez-Burgos MA, Salido GM, Adeghate E, and Singh J. Mechanism of exocrine pancreatic insufficiency in streptozotocin-induced type 1 diabetes mellitus. Ann N Y Acad Sci 1084: 71-88, 2006. PMID: 17151294.
- Patel R, Singh J, Yago MD, Vilchez JR, Martinez-Victoria E, and Manas M. Effect of insulin on exocrine pancreatic secretion in healthy and diabetic anaesthetised rats. Mol Cell Biochem 261: 105-110, 2004. PMDI: 15362492.
- Philippe MF, Benabadji S, Barbot-Trystram L, Vadrot D, Boitard C, and Larger E. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas 40: 359-363, 2011. PMID: 21283038.
- Pierzynowski S, and Barej W. The dependence of exocrine pancreatic secretion on insulin in sheep. Q J Exp Physiol 69: 35-39, 1984. PMID: 6201945.
- Pradayrol L, Jornvall H, Mutt V, and Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett 109: 55-58, 1980. PMID: 7353633.
- Putnam WS, Liddle RA, and Williams JA. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol 256: G698-703, 1989. PMID: 2565088.
- Rajaratnam SG, and Martin IG. Admission serum glucose level: an accurate predictor of outcome in gallstone pancreatitis. Pancreas 33: 27-30, 2006. PMID: 16804409.
- Rawla P, Sunkara T, Thandra KC, and Gaduputi V. Hypertriglyceridemia-induced pancreatitis: updated review of current treatment and preventive strategies. Clin J Gastroenterol 11: 441-448, 2018. PMID: 29923163.
- Reboud JP, Marchis-Mouren G, Cozzone A, and Desnuelle P. Variations in the biosynthesis rate of pancreatic amylase and chymotrypsinogen in response to a starch-rich or a protein-rich diet. Biochem Biophys Res Commun 22: 94-99, 1966. PMID: 5937345.
- Reisine T, and Bell GI. Molecular properties of somatostatin receptors. Neuroscience 67: 777-790, 1995. PMID: 7675204.
- Renner IG, Savage WT, 3rd, Pantoja JL, and Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci 30: 1005-1018, 1985. PMID: 3896700.
- Roberts SR, and Hamedani B. Benefits and methods of achieving strict glycemic control in the ICU. Critical Care Nursing Clinics 16: 537-545, 2004. PMID: 15571942.
- Rooman I, Lutz C, Pinho AV, Huggel K, Reding T, Lahoutte T, Verrey F, Graf R, and Camargo SM. Amino acid transporters expression in acinar cells is changed during acute pancreatitis. Pancreatology 13: 475-485, 2013. PMID: 24075511.
- Runzi M, Muller MK, Schmid P, von Schonfeld J, and Goebell H. Stimulatory and inhibitory effects of galanin on exocrine and endocrine rat pancreas. Pancreas 7: 619-623, 1992. PMID: 1280360.
- Rustagi S, Warner RR, and Divino CM. Serum pancreastatin: the next predictive neuroendocrine tumor marker. J Surg Oncol 108: 126-128, 2013. PMID: 23775817.
- Ryu GR, Sung CH, Kim MJ, Sung JH, Lee KH, Park DW, Sim SS, Min DS, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, and Jo AY. Changes in IP3 receptor are associated with altered calcium response to cholecystokinin in diabetic rat pancreatic acini. Pancreas 29: e106-112, 2004. PMID: 15502636.
- Sah RP, Nagpal SJ, Mukhopadhyay D, and Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 10: 423-433, 2013. PMID: 23528347.
- Saito A, Williams JA, and Kanno T. Potentiation by insulin of the acetylcholine-induced secretory response of the perfused rat pancreas. Biomedical Research 1: 101-103, 1980. DOI: 10.2220/biomedres.1.101.
- Saito A, Williams JA, and Kanno T. Potentiation of cholecystokinin-induced exocrine secretion by both exogenous and endogenous insulin in isolated and perfused rat pancreata. J Clin Invest 65: 777-782, 1980. PMID: 6987265.
- Sakamoto C, Goldfine ID, and Williams JA. The somatostatin receptor on isolated pancreatic acinar cell plasma membranes. Identification of subunit structure and direct regulation by cholecystokinin. J Biol Chem 259: 9623-9627, 1984. PMID: 6146617.
- Sakamoto C, Matozaki T, Nagao M, and Baba S. Coupling of guanine nucleotide inhibitory protein to somatostatin receptors on pancreatic acinar membranes. Am J Physiol Gastrointest Liver Physiol 253: G308-314, 1987. PMID: 2888315.
- Sakamoto C, Williams JA, Roach E, and Goldfine ID. In vivo localization of insulin binding to cells of the rat pancreas. Proc Soc Exp Biol Med 175: 497-502, 1984. PMID: 6369335.
- Samad A, James A, Wong J, Mankad P, Whitehouse J, Patel W, Alves-Simoes M, Siriwardena AK, and Bruce JI. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. J Biol Chem 289: 23582-23595, 2014. PMID: 24993827.
- Sankaran H, Iwamoto Y, Korc M, Williams JA, and Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol Gastrointest Liver Physiol 240: G63-68, 1981. PMID: 7006418.
- Sans MD. Measurement of pancreatic protein synthesis. Pancreapedia: Exocrine Pancreas Knowledge base, 2010. DOI: 10.3998/panc.2010.16.
- Sans MD, Amin RK, Vogel NL, D'Alecy LG, Kahn RC, and Williams JA. Specific deletion of insulin receptors on pancreatic acinar cells defines the insulin-acinar axis: implications for pancreatic insufficiency in diabetes. Gastroenterology 140: Suppl.1:A-233, 2011. ISSN: 0016-5085.
- Sans MD, and Williams JA. The mTOR Signaling Pathway and Regulation of Pancreatic Function. Pancreapedia: Exocrine Pancreas Knowledge base, 2017. DOI: 10.3998/panc.2017.08.
- Schmidt WE, and Creutzfeldt W. Pancreastatin--a novel regulatory peptide? Acta Oncol 30: 441-449, 1991. PMID: 1854501.
- Schnapp D, Reid CJ, and Harris A. Localization of expression of human beta defensin-1 in the pancreas and kidney. J Pathol 186: 99-103, 1998. PMID: 9875146.
- Schonfeld JV, Muller MK, Demirtas B, Soukup J, Runzi M, and Goebell H. Effect of neural blockade on somatostatin-induced inhibition of exocrine pancreatic secretion. Digestion 43: 81-86, 1989. PMID: 2572497.
- Schwartz TW. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 85: 1411-1425, 1983. PMID: 6138294.
- Seicean A, Tantau M, Grigorescu M, Mocan T, Seicean R, and Pop T. Mortality risk factors in chronic pancreatitis. J Gastrointestin Liver Dis 15: 21-26, 2006. PMID: 16680228.
- Shaw HM, and Heath TJ. The effect of glucagon on the formation of pancreatic juice and bile in the rat. Can J Physiol Pharmacol 51: 1-5, 1973. PMID: 4692196.
- Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, and Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem 270: 4412-4417, 1995. PMID: 7876206.
- Shiratori K, Lee KY, Chang TM, Jo YH, Coy DH, and Chey WY. Role of pancreatic polypeptide in the regulation of pancreatic exocrine secretion in dogs. Am J Physiol Gastrointest Liver Physiol 255: G535-541, 1988. PMID: 3189545.
- Shiratori K, and Shimizu K. Insulo-Acinar Relationship. In: The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery, edited by Beger HG, Warshaw AL, Hruban RH, Buchler MW, Lerch MM, Neoptolemos JP, Shimosegawa T, and Whitcomb DCJohn Wiley & Sons Ltd, p. 123-131, 2018. ISBN: 978-1-119-18839-1.
- Singer MV, Tiscornia OM, Mendes de Oliveiro JP, Demol P, Levesque D, and Sarles H. Effect of glucagon on canine exocrine pancreatic secretion stimulated by a test meal. Can J Physiol Pharmacol 56: 1-6, 1978. PMID: 638847.
- Singh J. Mechanism of action of insulin on acetylcholine-evoked amylase secretion in the mouse pancreas. J Physiol 358: 469-482, 1985. PMID: 2580088.
- Singh M. Amylase release from dissociated mouse pancreatic acinar cells stimulated by glucagon: effect of membrane stabilizers. J Physiol 309: 81-91, 1980. PMID: 6166745.
- Singh M. Effect of glucagon on digestive enzyme synthesis, transport and secretion in mouse pancreatic acinar cells. J Physiol 306: 307-322, 1980. PMID: 6162027.
- Singh M. Effect of somatostatin on amylase secretion from in vivo and in vitro rat pancreas. Dig Dis Sci 31: 506-512, 1986. PMID: 2421987.
- Singh P, Asada I, Owlia A, Collins TJ, and Thompson JC. Somatostatin inhibits VIP-stimulated amylase release from perifused guinea pig pancreatic acini. Am J Physiol Gastrointest Liver Physiol 254: G217-223, 1988. PMID: 2450469.
- Sjodin L, Holmberg K, and Lyden A. Insulin receptors on pancreatic acinar cells in guinea pigs. Endocrinology 115: 1102-1109, 1984. PMID: 6086285.
- Snook JT. Effect of diet, adrenalectomy, diabetes, and actinomycin D on exocrine pancreas. Am J Physiol 215: 1329-1333, 1968. PMID: 5722992.
- Sofrankova A. Effect of exogenous and endogenous insulin on the secretory response of the pancreas to the octapeptide of cholecystokinin (CCK8) in normal rats. Physiol Bohemoslov 33: 391-398, 1984. PMID: 6095348.
- Sofrankova A, and Dockray GJ. Cholecystokinin- and secretin-induced pancreatic secretion in normal and diabetic rats. Am J Physiol Gastrointest Liver Physiol 244: G370-374, 1983. PMID: 6301289.
- Soling HD, and Unger KO. The role of insulin in the regulation of -amylase synthesis in the rat pancreas. Eur J Clin Invest 2: 199-212, 1972. PMID: 5053831.
- Song F, Zhong LJ, Han L, Xie GH, Xiao C, Zhao B, Hu YQ, Wang SY, Qin CJ, Zhang Y, Lai DM, Cui P, and Fang XM. Intensive insulin therapy for septic patients: a meta-analysis of randomized controlled trials. Biomed Res Int 2014: 698265, 2014. PMID: 25136614.
- Studley JG, Mathie RT, and Blumgart LH. Blood flow measurement in the canine pancreas. J Surg Res 42: 101-115, 1987. PMID: 3543497.
- Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, and Diana J. Pancreatic beta-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 43: 304-317, 2015. PMID: 26253786.
- Sung CK, and Williams JA. Insulin and ribosomal protein S6 kinase in rat pancreatic acini. Diabetes 38: 544-549, 1989. PMID: 2653925.
- Susini C, Esteve JP, Bommelaer G, Vaysse N, and Ribet A. Inhibition of exocrine pancreatic secretion by somatostatin in dogs. Digestion 18: 384-393, 1978. PMID: 750262.
- Susini C, Esteve JP, Vaysse N, Pradayrol L, and Ribet A. Somatostatin 28: effects on exocrine pancreatic secretion in conscious dogs. Gastroenterology 79: 720-724, 1980. PMID: 7409390.
- Sutherland EW, and De Duve C. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem 175: 663-674, 1948. PMID: 18880761.
- Suzuki T, Naruse S, and Yanaihara N. The inhibitory effect of octreotide on exocrine pancreatic secretion in conscious dogs. Pancreas 8: 226-232, 1993. PMID: 8096338.
- Taborsky GJ, Jr., and Ensinck JW. Contribution of the pancreas to circulating somatostatin-like immunoreactivity in the normal dog. J Clin Invest 73: 216-223, 1984. PMID: 6140271.
- Takacs T, Hegyi P, Jarmay K, Czako L, Gog C, Rakonczay Z, Jr., Nemeth J, and Lonovics J. Cholecystokinin fails to promote pancreatic regeneration in diabetic rats following the induction of experimental pancreatitis. Pharmacol Res 44: 363-372, 2001. PMID: 11712866.
- Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, and Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91-119, 2014. PMID: 24388214.
- Taparel D, Esteve JP, Susini C, Vaysse N, Balas D, Berthon G, Wunsch E, and Ribet A. Binding of somatostatin to guinea-pig pancreatic membranes: regulation by ions. Biochem Biophys Res Commun 115: 827-833, 1983. PMID: 6138038.
- Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, and Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324: 476-478, 1986. PMID: 3537810.
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, and Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett 164: 124-128, 1983. PMID: 6197320.
- Taylor IL. Pancreatic polypeptide family: pancreatic polypeptide, neuropeptide Y, and peptide YY. In: Handbook of Physiology: The Gastrointestinal System II Section VI, Vol 2. 1989.
- Taylor IL, Solomon TE, Walsh JH, and Grossman MI. Pancreatic polypeptide. Metabolism and effect on pancreatic secretion in dogs. Gastroenterology 76: 524-528, 1979. PMID: 428706.
- Terzin V, Varkonyi T, Szabolcs A, Lengyel C, Takacs T, Zsori G, Stajer A, Palko A, Wittmann T, Palinkas A, and Czako L. Prevalence of exocrine pancreatic insufficiency in type 2 diabetes mellitus with poor glycemic control. Pancreatology 14: 356-360, 2014. PMID: 25278304.
- Trimble ER, Bruzzone R, and Belin D. Insulin resistance is accompanied by impairment of amylase-gene expression in the exocrine pancreas of the obese Zucker rat. Biochem J 237: 807-812, 1986. PMID: 2432875.
- Trimble ER, Rausch U, and Kern HF. Changes in individual rates of pancreatic enzyme and isoenzyme biosynthesis in the obese Zucker rat. Biochem J 248: 771-777, 1987. PMID: 3325041.
- Tsai A, Cowan MR, Johnson DG, and Brannon PM. Regulation of pancreatic amylase and lipase gene expression by diet and insulin in diabetic rats. Am J Physiol Gastrointest Liver Physiol 267: G575-583, 1994. PMID: 7524347.
- Tsuang W, Navaneethan U, Ruiz L, Palascak JB, and Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol 104: 984-991, 2009. PMID: 19293788.
- Tsuchida T, Ohnishi H, Tanaka Y, Mine T, and Fujita T. Inhibition of stimulated amylase secretion by adrenomedullin in rat pancreatic acini. Endocrinology 140: 865-870, 1999. PMID: 9927317.
- Unger RH, Eisentraut AM, McCall M, and Madison LL. Glucagon antibodies and an immunoassay for glucagon. J Clin Invest 40: 1280-1289, 1961. PMID: 13779204.
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, and Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 345: 1359-1367, 2001. PMID: 11794168.
- Varga G, Kisfalvi I, Jr., Kordas K, Wong H, Walsh JH, and Solomon TE. Effect of somatostatin immunoneutralization on gastric acid and pancreatic enzyme secretion in anesthetized rats. J Physiol Paris 91: 223-227, 1997. PMID: 9403799.
- Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, and Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem 279: 7199-7207, 2004. PMID: 14660681.
- Vaysse N, Chayvialle JA, Pradayrol L, Esteve JP, Susini C, Lapuelle J, Descos F, and Ribet A. Somatostatin 28: comparison with somatostatin 14 for plasma kinetics and low-dose effects on the exocrine pancreas in dogs. Gastroenterology 81: 700-706, 1981. PMID: 6114895.
- Vetterlein F, Petho A, and Schmidt G. Morphometric investigation of the microvascular system of pancreatic exocrine and endocrine tissue in the rat. Microvasc Res 34: 231-238, 1987. PMID: 3312966.
- von Schonfeld J, and Muller MK. The effect of pancreastatin on endocrine and exocrine pancreas. Scand J Gastroenterol 26: 993-999, 1991. PMID: 1947780.
- Vujasinovic M, Zaletel J, Tepes B, Popic B, Makuc J, Epsek Lenart M, Predikaka M, and Rudolf S. Low prevalence of exocrine pancreatic insufficiency in patients with diabetes mellitus. Pancreatology 13: 343-346, 2013. PMID: 23890131.
- Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, and Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5: 220-230, 2011. PMID: 20686513.
- Warzecha Z, Dembinski A, Ceranowicz P, Konturek SJ, Tomaszewska R, Stachura J, and Konturek PC. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J Physiol Pharmacol 54: 575-590, 2003. PMID: 14726612.
- Warzecha Z, Dembinski A, Konturek PC, Ceranowicz P, Konturek SJ, Tomaszewska R, Schuppan D, Stachura J, and Nakamura T. Hepatocyte growth factor attenuates pancreatic damage in caerulein-induced pancreatitis in rats. Eur J Pharmacol 430: 113-121, 2001. PMID: 11698071.
- Whitcomb DC, Puccio AM, Vigna SR, Taylor IL, and Hoffman GE. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res 760: 137-149, 1997. PMID: 9237528.
- Whitcomb DC, Taylor IL, and Vigna SR. Characterization of saturable binding sites for circulating pancreatic polypeptide in rat brain. Am J Physiol Gastrointest Liver Physiol 259: G687-691, 1990. PMID: 2221079.
- Wiener RS, Wiener DC, and Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300: 933-944, 2008. PMID: 18728267.
- Williams AJ, Chau W, Callaway MP, and Dayan CM. Magnetic resonance imaging: a reliable method for measuring pancreatic volume in Type 1 diabetes. Diabet Med 24: 35-40, 2007. PMID: 17227322.
- Williams JA, and Goldfine ID. The Insulin-Acinar Relationship. In: The Exocrine Pancreas: Biology, Pathobiology, and Diseases, edited by Go VLW et al. New York: Raven Press, 347-360, 1986.
- Williams JA. Amylase. Pancreapedia: Exocrine Pancreas Knowledge Base, 2019. DOI: 10.3998/panc.2019.02.
- Williams JA. Cholecystokinin (CCK) Regulation of Pancreatic Acinar Cells: Physiological Actions and Signal Transduction Mechanisms. Compr Physiol 9: 535-564, 2019. PMID: 30873601.
- Williams JA. Pancreatic Polypeptide. Pancreapedia: Exocrine Pancreas Knowledge Base, 2014. DOI: 10.3998/panc.2014.4.
- Williams JA, Bailey A, Humbel R, and Goldfine ID. Insulinlike growth factors bind to specific receptors in isolated pancreatic acini. Am J Physiol Gastrointest Liver Physiol 246: G96-99, 1984. PMID: 6320665.
- Williams JA, Bailey AC, Preissler M, and Goldfine ID. Insulin regulation of sugar transport in isolated pancreatic acini from diabetic mice. Diabetes 31: 674-682, 1982. PMID: 6761207.
- Williams JA, and Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 34: 980-986, 1985. PMID: 2412919.
- Wilson RM, Boden G, Shore LS, and Essa-Koumar N. Effect of somatostatin on meal-stimulated pancreatic exocrine secretions in dogs. Diabetes 26: 7-10, 1977. PMID: 830567.
- Wizemann V, Weppler P, and Mahrt R. Effect of glucagon and insulin on the isolated exocrine pancreas. Digestion 11: 432-435, 1974. PMID: 4463136.
- Yagihashi S. Exocrine pancreas of streptozotocin-diabetes rats treated with insulin. Tohoku J Exp Med 120: 31-42, 1976. PMID: 134471.
- Yamada T, Shojima N, Hara K, Noma H, Yamauchi T, and Kadowaki T. Glycemic control, mortality, secondary infection, and hypoglycemia in critically ill pediatric patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med 43: 1427-1429, 2017. PMID: 28424848.
- Yamada T, Shojima N, Noma H, Yamauchi T, and Kadowaki T. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med 43: 1-15, 2017. PMID: 27637719.
- Yatabe T, Inoue S, Sakaguchi M, and Egi M. The optimal target for acute glycemic control in critically ill patients: a network meta-analysis. Intensive Care Med 43: 16-28, 2017. PMID: 27686353.
- Young AA, Jodka C, Pittner R, Parkes D, and Gedulin BR. Dose-response for inhibition by amylin of cholecystokinin-stimulated secretion of amylase and lipase in rats. Regul Pept 130: 19-26, 2005. PMID: 15982756.
- Zechner D, Spitzner M, Bobrowski A, Knapp N, Kuhla A, and Vollmar B. Diabetes aggravates acute pancreatitis and inhibits pancreas regeneration in mice. Diabetologia 55: 1526-1534, 2012. PMID: 22327285.
- Zeggari M, Viguerie N, Susini C, Esteve JP, Vaysse N, Rivier J, Wunsch E, and Ribet A. Characterization of pancreatic somatostatin binding sites with a 125I-somatostatin 28 analog. Peptides 7: 953-959, 1986. PMID: 2882495.
- Zhang H, Kandil E, Lin YY, Levi G, and Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol 39: 870-881, 2004. PMID: 15513386.
- Zhou ZG, Gao XH, Wayand WU, Xiao LJ, and Du Y. Pancreatic microcirculation in the monkey with special reference to the blood drainage system of Langerhans islets: light and scanning electron microscopic study. Clin Anat 9: 1-9, 1996. PMID: 8838272.
- Zipf WB, O'Dorisio TM, Cataland S, and Sotos J. Blunted pancreatic polypeptide responses in children with obesity of Prader-Willi syndrome. J Clin Endocrinol Metab 52: 1264-1266, 1981. PMID: 7014602.
- Zyznar ES, Pietri AO, Harris V, and Unger RH. Evidence for the hormonal status of somatostatin in man. Diabetes 30: 883-886, 1981. PMID: 6115787.