Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2012.5
| Attachment | Size |
|---|---|
| 96.53 KB | |
| 472.18 KB |
Gene Symbol: Src
Alternate Names: Src, c-Src, pp60-Src
1. General Information
The discovery of Src family of non-receptor tyrosine kinases dates back a century ago (1911) when Peyton Rous noted tumors transmissible in chicken via cell free extracts (37). This eventually culminated in the discovery of the Rous Sarcoma virus gene product which was subsequently shown to cause tyrosine phosphorylation of proteins (15). V-Src i.e. the viral protein was thus the first described proto-oncogene, and c-Src is its normal cellular homologue in animals (41). Src family members play key roles in cell morphology, endocytosis, motility, proliferation and survival. There are 11 members in the family (Blk, Brk, Fgr, Frk, Fyn, Hck, Lck, Lyn, c-Src, Srm, and Yes) of which some are expressed in pancreatic acinar cells.
Structure and Regulation
The Src family kinases are about 60 KD in size and human c-Src has 536 amino acids, with the 3 additional amino acids inserted at the N terminus compared to chicken Src (533 amino acids). Src has a myristolyation sequence at the N terminus followed by 4 SH (Src homology) domains. The first one of which, i.e. the SH4 unique domain is 80 amino acids followed by a SH3 (81-145), a SH2 (151-248), a protein kinase (SH1) domain (273-523) and a C terminus sequence with intervening linker regions (Figure 1). The two established sites of regulation of are tyrosine 527 (Y527) located 6 amino acids from the C-terminus and Tyrosine 416 (Y416).
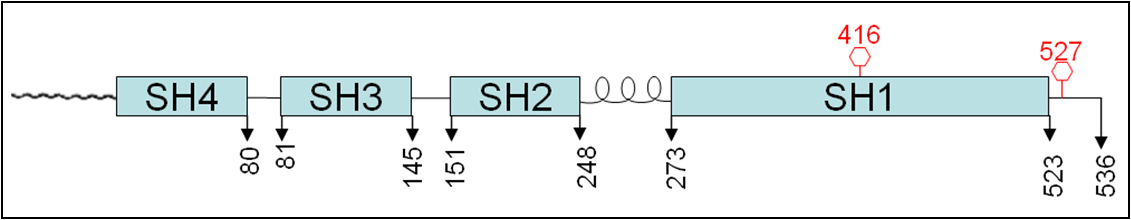
Figure 1: Diagram showing Domain structure of Src. The numbers indicate amino acids numbered starting from the N terminus. The wavy line preceding the SH4 domain is the 14 carbon myristolyation sequence. The proline rich domain (PRD) is indicated by the left handed helix connecting the SH2 and SH1 domains. The tyrosine phopshorylation sites are indicated by a red line with the hexagon.
The SH1 kinase domain of Src is bilobed and has an ATP binding region at the amino portion and a protein substrate binding area on the carboxy portion. The carboxy portion contains the activation loop with tyrosine 416, the phopshorylation of which stabilizes the active conformation. The functions of each domain are shown in table 1. For more detail the reader is referred to more extensive, excellent reviews on the structure and regulation of Src (4, 35, 36, 47).

Table 1: Table showing functions of the various domains of Src.
2. Pancreatic Information
Localization And Binding
Src normally shows a membraneous location in acinar cells, with apical enrichment under normal conditions and is associated with cortactin (39) (Figure 2). Activation of Src by supraphysiologic caerulein changes the staining pattern of Src and Yes to a cytosolic one along with its dissociation from cortactin (39). This activation also increases the binding of Src to RhoA (26) and the P85 subunit of PI3 kinases (25).

Figure 2: Src and Cortactin normally co-localize apically in acinar cells and localize differentially with supraphysiologic stimulation: Immunostaining of Src (A-C) and Cortactin (D-F) in acinar cells under control conditions (A, D) shows both to localize apically (Arrows). Treatment 10nM caerulein for 30 minutes (B, E). Results in a diffuse cytosolic appearance of Src (B) while cortactin localizes on the basal surface (arrowheads, E). The Src inhibitor PP2 prevents these caerulein induced changes, retaining both on the apical surface (C, F). Modified from Mol Biol Cell. 2008 May;19(5):2339-47.
Activation
Numerous studies (2, 13, 19, 22, 30, 31, 33, 34) have shown that diverse stimuli activate Src in pancreatic acinar cells. Yes and Lyn are both know to be activated by supraphysiologic doses of CCK (22, 30, 39). However Lyn is also shown to be activated by physiologic doses of CCK, EGF, and phorbol ester (30). Activation occurs rapidly in response to caerulein, with maximal phosphorylation of Y416 detected within 1-2 minutes (30, 39). Some studies suggest that Src activation may be calcium dependent (52).
Src family members have been hypothesized to play several roles in acinar cells including the regulation of store mediated calcium entry (34) by c-Src, changes in actin localization by Yes (19, 22), secretion by Src and Yes (22, 25), activation of PKC-delta by Lyn (45), upregulation of chemokine production in response to substance P via the neurokinin-1 receptor (33), endocytosis (13, 31), and acinar cell blebbing via Yes mediated phosphorylation of cortactin (39). Pharmacologic inhibition of Src results in a reduction in the severity of caerulein induced pancreatitis in both rats (39) and mice (33). Acinar phenomena in which the role of Src has been studied in detail are discussed below.
Calcium Homeostasis
Redondo et al showed that depletion of the intracellular calcium stores with thapsigargin induces Src activation (34). This is dependent on the integrity of the actin cytoskeleton, since it was prevented by cytochalasin D, which prevents actin polymerization. Conversely the Src inhibitor PP1 dose dependently reduced store mediated calcium entry (34). Tsunoda et al using a kinase assay showed that substrate phosphorylation was reduced in extracts from acini incubated in the presence of the extracellular calcium chelator EGTA (51). The exact mechanism by which Src is regulated by calcium or vice versa remains to be explored.
Actin Localization and Blebbing
Pancreatic acinar cells normally have filamentous actin (F-actin) enriched in the subapical area (27, 28, 39, 40). This reorganizes, with an increase on the basolateral surface, when acinar cells are stimulated with supraphysiologic doses of caerulein (1, 39, 40, 48, 49). Acinar blebbing induced by supraphysiologic caerulein is dependent on the actin cytoskeleton (48). The Src family member Yes has been thought to play a role in basolateral reorganization of F-actin (22, 39). Lutz et al showed that the Src inhibitor PP1 partially prevented actin changes (22). We have shown Src dependent tyrosine phosphorylation of the protein cortactin which regulates the branching of actin (39). Preventing cortactin phosphorylation, by pharmacologic inhibition of Src using PP2 or SU6656, or transfection of acini with a mutant cortactin which cannot be tyrosine phosphorylated by Src reduced baso-lateral reorganization of actin induced by supraphysiologic caerulein. Additionally, the pathological blebbing that is induced by supraphysiologic caerulein was also prevented by the mutant cortactin and pharmacologic inhibition of Src.
Pancreatic Cancer
Studies in pancreatic cancer have shown Src to be involved in tumorigenesis, cell proliferation, invasion, and motility. C-Src and oncogenic RAS have been shown to co-operatively initiate and accelerate pancreatic cancer (38). Aberrant acinar cell expression of the CCK2 receptor under the elastase promoter was associated with Src activation, formation of preneoplastic lesions and pancreatic tumor development (11). Src dependent activation of phosphatidyl inositol-3 kinase and p38 MAPK have been shown to be involved in expression of receptors for vascular endothelial growth factors (VEGF) and the angiogenic potential of pancreatic cancer (43).
Endocytosis, vesicular transport through the Golgi in pancreatic cancer cells is known to be regulated via phosphorylation of the large GTPase dynamin-2 (55) and its associated actin-binding protein, cortactin (5). Phosphorylation of Dynamin-2 at tyrosines 231, 579 by Src has also been shown to be involved in the metastatic migration and invasion of pancreatic tumor cells lines (10).
Pharmacologic inhibition of Src has been shown to inhibit progression and metastasis in orthotopic (50) and transgenic (23) models of pancreatic cancer. The Src inhibitor Dasatinib (BMS-354825) resulted in decreased phosphorylation of extracellular signal-regulated kinase (ERK), and mitogen-activated protein kinase (MAPK), focal adhesion kinase (FAK), paxillin, AKT, signal transducers and activators of transcription 3 (STAT3), as well as decreased cyclin D1 expression. This prevented anchorage-independent growth, proliferation, migration, invasion, cell cycle progression while stimulating apoptosis (24).
3. Tools for studying Src in acinar cells
a. Antibodies
Src family: (SC-18, Santa Cruz biotechnology) Polyclonal antibody, use 1:500 for WB.
Phospho-Src (Tyr416): (catalog # 2101, Cell Signal), Rabbit polyclonal antibody raised against a phosphopeptide; use 1:1000 for WB.
b. Inhibitors
Pharmacologic inhibition of Src has is indispensable in determining Src’s function in various acinar biologic processes. This remains the main approach for phenomena such as trypsinogen activation that significantly diminish in cultured acinar cells or are not replicated by exocrine cell lines such as AR42J cells.
The Src inhibitors used include the pyrazolo-pyrimidine compound PP1 (16) (4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine), and the related PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine) which act as competitive inhibitors of ATP binding. However PP1 has been shown to inhibit PDGF-β receptor (53) directly along with inhibiting Ret (6), c-Kit and Bcr-Abl (46). SU6656 (2-oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid dimethylamide), was synthesized as a more specific inhibitor of Src (3). The IC50 of all these agents for various Src family members (except PP1 which has an IC50 of 6nM for Lyn) ranges from 20-280nM (29). Dasatinib (BMS-354825; N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide) inhibits both the Src family kinases and Bcr-Abl with an IC50 of < 1nM (8). Despite the other targets PP1, PP2, and Dasatinib when used at appropriate concentrations for short term studies remain invaluable, since c-Abl is not normally expressed as a protein in acinar cells (unpublished data). However caution may need to be exercised in studies pertaining to PDGF since PDGFR-β is expressed in acinar cells and the levels of both PDGFR-β and its ligand are increased in chronic disease (9). Further relevant details of inhibitors are available in excellent reviews (7, 29, 44).
c. Activation
The commonly used methods for studying Src activation are:
- Immuno-precipitation and western blotting for active Src (PY416).
- Src kinase activity assays.
Src Kinase assays have been used previously in acinar cells (22, 51). These are quantitative, and can be done on both cell lysates and immuno precipitated Src family members. Various substrates (e.g. PKS2-biotin substrate, p34cdc2 [6-20]) have been used by different groups (22, 51). We (39) and others (30) have used the widely published method of determining the amount of active Src. This is described elsewhere.
d. Mouse Lines
While pharmacologic inhibition of Src has proven to be beneficial in rat caerulein pancreatitis (39), the role of individual Src family members in pancreatitis remains to be determined. While there are redundancies in their functions, triple knock outs of Src, Yes and Fyn are embryonically lethal (18). Studies of mice genetically deficient in individual Src family members suggest their role in several of the mechanisms relevant to pancreatitis. For example c-Src-/- mice have reduced vascular permeability which minimizes the damage resulting from ischemia reperfusion injury (32) such as in stroke or myocardial infarction (54). Lyn -/- mice have defects in immunoglobulin-mediated signaling, suggesting that it has a role in establishing B cell tolerance (17). While genetic knock outs of Fyn display defects in T cell signaling (42), dual knock outs for Hck, Fgr display defects in innate immunity as evidenced by (20, 21) defective neutrophil adhesion and migration and macrophages from triple knock outs of Hck, Fgr and Lyn display defects in Fcgamma receptor-mediated phagocytosis (12).
4. Summary
While some Src family members such as Yes have been implicated in specific functions of acinar cells including actin dynamics, and the role of Src in pancreatic carcinogenesis, growth and invasion is being explored; the roles other Src family members may play in acinar cell physiology or diseases such as pancreatic cancer, acute pancreatitis remains to be determined.
5. References
- Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, and Adler G. Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis. American Journal of Physiology 282: G450-460, 2002. PMID: 11841995
- Berna MJ, Tapia JA, Sancho V, Thill M, Pace A, Hoffmann KM, Gonzalez-Fernandez L, and Jensen RT. Gastrointestinal growth factors and hormones have divergent effects on Akt activation. Cellular Signalling 21: 622-638, 2009. PMID: 19166928
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, and Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20: 9018-9027, 2000. PMID: 11074000
- Boggon TJ, and Eck MJ. Structure and regulation of Src family kinases. Oncogene 23: 7918-7927, 2004. PMID: 15489910
- Cao H, Chen J, Krueger EW, and McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the "constitutive" endocytosis of transferrin. Mol Cell Biol 30: 781-792, 2010. PMID: 19995918
- Carlomagno F, Vitagliano D, Guida T, Napolitano M, Vecchio G, Fusco A, Gazit A, Levitzki A, and Santoro M. The kinase inhibitor PP1 blocks tumorigenesis induced by RET oncogenes. Cancer Research 62: 1077-1082, 2002. PMID: 11861385
- Chong YP, Ia KK, Mulhern TD, and Cheng HC. Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases. Biochimica Biophysica Acta 1754: 210-220, 2005. PMID: 16198159
- Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, and Barrish JC. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem 49: 6819-6832, 2006. PMID: 17154512
- Ebert M, Kasper HU, Hernberg S, Friess H, Buchler MW, Roessner A, Korc M, and Malfertheiner P. Overexpression of platelet-derived growth factor (PDGF) B chain and type beta PDGF receptor in human chronic pancreatitis. Digestive Diseases and Sciences 43: 567-574, 1998. PMID: 9539653
- Eppinga RD, Krueger EW, Weller SG, Zhang L, Cao H, and McNiven MA. Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene. PMID: 21841817
- Ferrand A, Vatinel S, Kowalski-Chauvel A, Bertrand C, Escrieut C, Fourmy D, Dufresne M, and Seva C. Mechanism for Src activation by the CCK2 receptor: Patho-physiological functions of this receptor in pancreas. World J Gastroenterol 12: 4498-4503, 2006. PMID: 16874861
- Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, and Lowell CA. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Medicine 191: 669-682, 2000. PMID: 10684859
- Freedman SD, Katz MH, Parker EM, and Gelrud A. Endocytosis at the apical plasma membrane of pancreatic acinar cells is regulated by tyrosine kinases. Am J Physiol 276: C306-311, 1999. PMID: 9950757
- Garcia LJ, Rosado JA, Gonzalez A, and Jensen RT. Cholecystokinin-stimulated tyrosine phosphorylation of p125FAK and paxillin is mediated by phospholipase C-dependent and -independent mechanisms and requires the integrity of the actin cytoskeleton and participation of p21rho. Biochem J 327 ( Pt 2): 461-472, 1997. PMID: 9359417
- Gilmer TM, and Erikson RL. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature 294: 771-773, 1981. PMID: 6172716
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, and Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271: 695-701, 1996. PMID: 8557675
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, and Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83: 301-311, 1995. PMID: 7585947
- Klinghoffer RA, Sachsenmaier C, Cooper JA, and Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 18: 2459-2471, 1999. PMID: 10228160
- Leser J, Beil MF, Musa OA, Adler G, and Lutz MP. Regulation of adherens junction protein p120(ctn) by 10 nM CCK precedes actin breakdown in rat pancreatic acini. Am J Physiol 278: G486-491, 2000. PMID: 10712269
- Lowell CA, and Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc Natl Acad Sci USA 95: 7580-7584, 1998. PMID: 9636192
- Lowell CA, Fumagalli L, and Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol 133: 895-910, 1996. PMID: 8666673
- Lynch G, Kohler S, Leser J, Beil M, Garcia-Marin LJ, and Lutz MP. The tyrosine kinase Yes regulates actin structure and secretion during pancreatic acinar cell damage in rats. Pflugers Arch 447: 445-451, 2004. PMID: 14634819
- Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, Doyle B, McKay C, Heung MY, Oien KA, Frame MC, Evans TR, Sansom OJ, and Brunton VG. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139: 292-303. PMID: 20303350
- Nagaraj NS, Smith JJ, Revetta F, Washington MK, and Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Molecular cancer therapeutics 9: 2322-2332. PMID: 20682659
- Nozu F, Owyang C, and Tsunoda Y. Involvement of phosphoinositide 3-kinase and its association with pp60src in cholecystokinin-stimulated pancreatic acinar cells. Eur J Cell Biol 79: 803-809, 2000. PMID: 11139143
- Nozu F, Tsunoda Y, Ibitayo AI, Bitar KN, and Owyang C. Involvement of RhoA and its interaction with protein kinase C and Src in CCK-stimulated pancreatic acini. Am J Physiol 276: G915-923, 1999. PMID: 10198335
- O'Konski MS, and Pandol SJ. Cholecystokinin JMV-180 and caerulein effects on the pancreatic acinar cell cytoskeleton. Pancreas 8: 638-646, 1993. PMID: 7508112
- O'Konski MS, and Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest 86: 1649-1657, 1990. PMID: 1700797
- Okutani D, Lodyga M, Han B, and Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol 291: L129-141, 2006. PMID: 16581827
- Pace A, Tapia JA, Garcia-Marin LJ, and Jensen RT. The Src family kinase, Lyn, is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors which stimulate its association with numerous other signaling molecules. Biochimica Biophysica Acta 1763: 356-365, 2006. PMID: 16713446
- Parker EM, Zaman MM, and Freedman SD. GP2, a GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas 21: 219-225, 2000. PMID: 11039464
- Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, and Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nature Medicine 7: 222-227, 2001. PMID: 11175854
- Ramnath RD, Sun J, and Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharmacol Exp Therapeut 329: 418-428, 2009. PMID: 19211920
- Redondo PC, Lajas AI, Salido GM, Gonzalez A, Rosado JA, and Pariente JA. Evidence for secretion-like coupling involving pp60src in the activation and maintenance of store-mediated Ca2+ entry in mouse pancreatic acinar cells. Biochemical Journal 370: 255-263, 2003. PMID: 12423207
- Roskoski R, Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Research Commun 331: 1-14, 2005. PMID: 15845350
- Roskoski R, Jr. Src protein-tyrosine kinase structure and regulation. Biochem Biophys Research Commun 324: 1155-1164, 2004. PMID: 15504335
- Rous P. A Sarcoma of the Fowl Transmissible by an Agent Separable from the Tumor Cells. J Exp Med 13: 397-411, 1911. PMID: 19867421
- Shields DJ, Murphy EA, Desgrosellier JS, Mielgo A, Lau SK, Barnes LA, Lesperance J, Huang M, Schmedt C, Tarin D, Lowy AM, and Cheresh DA. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene 30: 2123-2134. PMID: 21242978
- Singh VP, and McNiven MA. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell 19: 2339-2347, 2008. PMID: 18353971
- Singh VP, Saluja AK, Bhagat L, Hietaranta AJ, Song A, Mykoniatis A, Van Acker GJ, and Steer ML. Serine protease inhibitor causes F-actin redistribution and inhibition of calcium-mediated secretion in pancreatic acini. Gastroenterology 120: 1818-1827, 2001. PMID: 11375962
- Smart JE, Oppermann H, Czernilofsky AP, Purchio AF, Erikson RL, and Bishop JM. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci USA 78: 6013-6017, 1981. PMID: 6273838
- Stein PL, Lee HM, Rich S, and Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70: 741-750, 1992. PMID: 1387588
- Summy JM, Trevino JG, Baker CH, and Gallick GE. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas 31: 263-274, 2005. PMID: 16163059
- Susa M, and Teti A. Tyrosine kinase src inhibitors: potential therapeutic applications. Drug News & Perspectives 13: 169-175, 2000. PMID: 12937607
- Tapia JA, Garcia-Marin LJ, and Jensen RT. Cholecystokinin-stimulated protein kinase C-delta kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem 278: 35220-35230, 2003. PMID: 12842900
- Tatton L, Morley GM, Chopra R, and Khwaja A. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J Biol Chem 278: 4847-4853, 2003. PMID: 12475982
- Thomas SM, and Brugge JS. Cellular functions regulated by Src family kinases. Ann Rev Cell Develop Biol 13: 513-609, 1997. PMID: 9442882
- Torgerson RR, and McNiven MA. The actin-myosin cytoskeleton mediates reversible agonist-induced membrane blebbing. J Cell Sci 111 ( Pt 19): 2911-2922, 1998. PMID: 9730983
- Torgerson RR, and McNiven MA. Agonist-induced changes in cell shape during regulated secretion in rat pancreatic acini. J Cell Physiol 182: 438-447, 2000. PMID: 10653611
- Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, and Gallick GE. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168: 962-972, 2006. PMID: 16507911
- Tsunoda Y, Yoshida H, Africa L, Steil GJ, and Owyang C. Src kinase pathways in extracellular Ca(2+)-dependent pancreatic enzyme secretion. Biochem Biophys Res Commun 227: 876-884, 1996. PMID: 8886024
- Tsunoda Y, Yoshida H, and Nozu F. Receptor-operated Ca2+ influx and its association with the Src family in secretagogue-stimulated pancreatic acini. Biochem Biophys Res Commun 314: 916-924, 2004. PMID: 14741724
- Waltenberger J, Uecker A, Kroll J, Frank H, Mayr U, Bjorge JD, Fujita D, Gazit A, Hombach V, Levitzki A, and Bohmer FD. A dual inhibitor of platelet-derived growth factor beta-receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells. A novel candidate for prevention of vascular remodeling. Circ Res 85: 12-22, 1999. PMID: 10400906
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, and Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 113: 885-894, 2004. PMID: 15067321
- Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, and McNiven MA. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc Natl Acad Sci USA 107: 5863-5868, 2010. PMID: 20231454


