Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2013.13
| Attachment | Size |
|---|---|
| 650.61 KB |
Introduction
In 1961, Sarles et al reported a case of pancreatitis with hypergammaglobulinemia, which in retrospect appears to be identical to autoimmune pancreatitis (AIP) (26). In 1995, Yoshida et al. described such a case as AIP (37). In 2001, Hamano et al. later reported increased serum levels of IgG4 in AIP (9). The histopathological findings of AIP are characterized by the periductal localization of predominantly CD4 positive T-cells, IgG4-positive plasma cells, storiform fibrosis with acinar cell atrophy frequently resulting in the stenosis of the main pancreatic duct, and obliterative fibrosis, resulting in the so called lymphoplasmacytic sclerosing pancreatitis (LPSP) (13, 22, 23, 27). Although the infiltration of IgG4-positive cells and increased serum levels of IgG4 are characteristic, they are not specific for type 1 AIP and the role of IgG4 in the development of AIP and IgG4-related disease remains unclear (10, 34, 35).
Immunology of immunoglobulin subclasses
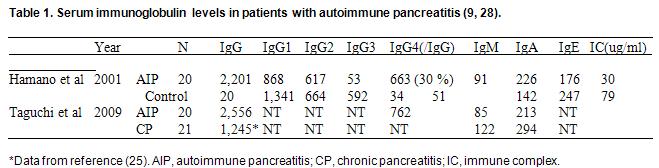
Generally, the amount of IgG4 does not vary with sex or age, and the quantity of IgG4 as well as the IgG4/total IgG ratio tends to remain constant (25). In normal subjects, IgG4 consists of 4–6% of total IgG, and its serum elevation has been seen in several conditions, such as allergic disease, parasite infection, and pemphigus vulgaris (25). In type 1 AIP, total IgG, IgG1, IgG2, IgG4 and IgE were usually increased compared with healthy subjects, while IgM, IgA, and the ratios of IgG to IgM or IgA, are decreased compared with normal or other control diseases (9, 28) (Table 1). In AIP, all subclasses of IgG were increased compared with other types of pancreatitis.
Although the association with IgE-mediated allergy and IgG4 antibodies is well-known, the characteristics of IgG4 are less understood (24). IgG4 antibodies participate in a continuous process referred to as Fab-arm exchange, which describes swapping a heavy chain and attached lightchain (half-molecule) with a heavy-light chain pair from anothermolecule (36). This results in asymmetric antibodies with two different antigen-combining sites. While these modified antibodies are hetero- bivalent, they behave as monovalemt antibodies (Figure 1A) (36). Another aspect of IgG4 mimics IgG rheumatoid factor (RF) activity by interacting with IgG on a solid support (Figure 1B) (11). In contrast to conventional RF, which binds via its variable domains, the activity of IgG4 is located in its constant domains, but is inefficient to activate potentially dangerous effector systems due to its low affinity for C1q and the classical Fcγ-receptors.
Comparison of various markers in differentiating between AIP and pancreatic cancer showed that the best results are obtained using IgG4, which has 86% sensitivity, 96% specificity, and 91% accuracy (Table 2) (12). IgG4 was therefore adopted as the best marker in the diagnostic criteria of type 1 AIP (12). However, serum IgG4 elevation or numerous IgG4-bearing plasma cell infiltrations has been reported in some patients with pancreatic cancer, suggesting that these features are not completely specific for AIP and cannot exclude the presence of pancreatic cancer (7).
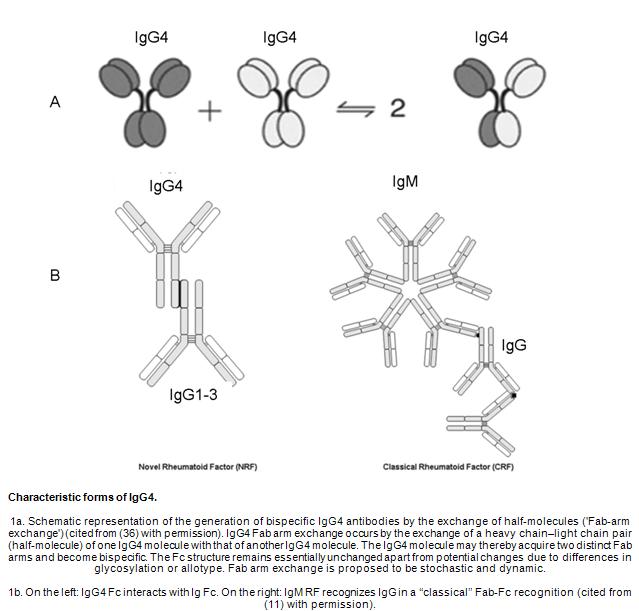

The complement system
Patients with active AIP occasionally have decreased complement (C3, C4) levels with elevated circulating immune complexes and serum IgG4 elevation (4, 9). However, a recent study showed that the classical pathway of complement activation through IgG1 may be involved in the development of AIP rather than mannose-binding lectin or alternative pathways through IgG4 (16). Moreover, IgG4 bound to other isotypes such as IgG1, IgG2, and IgG3 with an Fc-Fc interaction develop immune complexes in patients with AIP. In this setting IgG4 may contribute to the clearance of immune complexes or termination of the inflammatory process by preventing the formation of large immune complexes by blocking the Fc mediated effector functions of IgG1 (11). Compared with SLE, tubulointerstitial nephritis (TIN) is more often observed in renal lesions of IgG4-related disease. In TIN associated with AIP deposition of immune complex (IgG and C3) is more commonly observed in the tubular basement membrane, rather than the in the glomerular basement membrane as is typically seen in SLE (32).
Autoantibodies
In addition to increased total IgG and IgG4, patients with IgG4-related disease often have detectable autoantibodies, albeit not organ-specific (22,23). Although some patients with IgG4-related disease have non-specific antibodies such as anti-nuclear antibody (ANA), there is no clear association, aside from overlapping symptoms, between IgG4-related disease and common autoimmune diseases such as Sjögren’s syndrome and SLE. In regards to IgG4 function, it remains unclear if IgG4-related disease is a true autoimmune or allergic disease. However, the frequent coexistence of other organ involvement lead to the concept that there may be common target antigens in the involved organs such as the pancreas, salivary gland, biliary tract, lung, renal tubules, etc.. Although the disease specific antibodies have not been identified several disease-related antibodies such as anti-lactoferrin (LF) (20,30), anti-carbonic anhydrase (CA)-II (2, 17, 20, 30), anti-CA-IV (19), anti-pancreatic secretory trypsin inhibitor (PSTI) (3), anti-amylase-alpha (5), anti-HSP-10 (29), and anti-plasminogen-binding protein (PBP) peptide autoantibodies (6) have been reported. Although patients have increased serum levels of IgG4, the major subclass of these autoantibodies is not necessarily IgG4, but often IgG1 (3). CA-II (20), CA-IV (19), LF (20) and PSTI (3) are distributed in the ductal cells of several exocrine organs, including the pancreas, salivary gland, biliary duct, and lung (17,20). Although all peptides have not been systematically studied,
immunization with CA-II or LF induced systemic lesions such as pancreatitis, sialadenitis, cholangitis, and interstitial nephritis in mice models appear similar to human IgG4-related diseases (18, 33). The high prevalence of these antibodies suggests that they are at least potential candidate target antigens in AIP (20, 30).
Molecular mimicry among microbes and target antigens may be a possible mechanism to overcome immune tolerance. This hypothesis is based on the concept that infectious agents share one or more epitopes with self-components, or infectious agents cause bystander activation of immune cells with autoaggressive potential (8,14,15). Guarneri and colleagues showed significant homology between human CA-II and alpha-CA of H. pylori, a fundamental enzyme for bacterial survival and proliferation in the stomach (8). Moreover, the homologous segments contain the binding motif of DRB1*0405, which confers a risk for AIP development (8). The PBP peptide identified in European patients with AIP shows homology with an amino acid sequence of PBP of H. pylori and with ubiquitin-protein ligase E3 component n-recognin 2 (UBR2), an enzyme highly expressed in acinar cells of the pancreas (6). These findings suggest that gastric H. pylori infection might trigger AIP in genetically predisposed subjects (8, 14, 15).
Diabetes mellitus is affects 43~68% of the patients with AIP, but autoantibodies against glutamic acid decarboxylase, beta-cell or tyrosine phosphatase-like protein associated type1 DM are rarely observed(21). These findings suggest that islet cells are not likely targeted in the development of DM associated with AIP.
Summary
Although serum IgG4 elevation is a characteristic finding and useful to establish a diagnosis of type 1 AIP, it is not specific for this disorder. Importantly it can be seen in other conditions with similar clinical presentations, including pancreatic cancer. The role of IgG4 antibodies in the pathogenesis of AIP and IgG4-related disease remains unclear. Several auto-antibodies have been identified in subjects with AIP, but additional studies are needed to clarify the significance of these findings in the pathophysiology of AIP.
Acknowledgment
This study was partly supported by Grant-in-Aid for Scientific Research of Ministry of Culture and Science of Japan (23591017), grants-in-aid from CREST Japan Science and Technology Agency, and Grant-in-Aid for “Research for Intractable Disease” Program from the Ministry of Health, Labour and Welfare of Japan.
References
- Aoki S, Nakazawa T, Ohara H, Sano H, Nakao H, Joh T, Murase T, Eimoto T, Itoh M. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients’ sera. Histopathology. 47147–58, 2005. PMID: 16045775
- Aparisi L, Farre A, Gomez-Cambronero L, Martinez J, De Las Heras G, Corts J, Navarro S, Mora J, Lopez-Hoyos M, Sabater L, Ferrandez A, Bautista D, Perez-Mateo M, Mery S, Sastre J. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 54: 703-9, 2005. PMID: 15831920
- Asada M, Nishio A, Uchida K, Kido M, Ueno S, Uza N, Kiriya K, Inoue S, Kitamura H, Ohashi S, Tamaki H, Fukui T, Matsuura M, Kawasaki K, Nishi T, Watanabe N, Nakase H, Chiba T, Okazaki K. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 33(1): 20-6, 2006. PMID: 16804408
- Cornell L D, Chicano S L, Deshpande V, Collins AB, Selig MK, Lauwers GY, Barisoni L, Colvin RB. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol 31: 1586-97,2007. PMID: 17895762
- Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, Kobayashi T. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 58: 732-7, 2009. PMID: 19001184
- Frulloni L, Lunardi C, Simone R, Dolcino M, Scattolini C, Falconi M, Benini L, Vantini I, Corrocher R, Puccetti A. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med 361: 2135-42, 2009. PMID: 19940298
- Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 inthe diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 102:1646–53, 2007. PMID: 17555461
- Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med 9: 741-4,2005. PMID: 16202223
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K.High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344: 732-8, 2001. PMID: 11236777
- Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol; 41: 613-25, 2006. PMID: 16932997
- Kawa S, Kitahara K, Hamano H, Ozaki Y, Arakura N, Yoshizawa K, Umemura T, Ota M, Mizoguchi S, Shimozuru Y, Bahram S. A novel immunoglobulin-immunoglobulin interaction in autoimmunity. PLoS One 3: e1637, 2008. PMID: 18297131
- Kawa S, Okazaki K, Kamisawa T, Shimosegawa T, Tanaka M. Working members of Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society. Japanese consensus guidelines for management of autoimmune pancreatitis: II. Extrapancreatic lesions, differential diagnosis. J Gastroenterol 45:355–369, 2010. PMID: 20127119
- Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol;22: 387-95, 199. PMID: 2050373
- Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med 9: 196-207, 2005. PMID: 15784177
- Kountouras J, Zavos C, Gavalas E, Tzilves D. Challenge in the pathogenesis of autoimmune pancreatitis: potential role of helicobacter pylori infection via molecular mimicry. Gastroenterology 133: 368-9, 2007. PMID: 17631165
- Muraki T, Hamano H, Ochi Y, Komatsu K, Komiyama Y, Arakura N, Yoshizawa K, Ota M, Kawa S, Kiyosawa K. Autoimmune pancreatitis and complement activation system. Pancreas 32: 16-21, 2006. PMID: 16340739
- Nishi H, Tojo A, Onozato M L, Jimbo R, Nangaku M, Uozaki H, Hirano K, Isayama H, Omata M, Kaname S, Fujita T. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrol Dial Transplant 22: 1273-5,2007. PMID: 17138573
- Nishimori I, Bratanova T, Toshkov I, Caffrey T, Mogaki M, Shibata Y, Hollingsworth MA. Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol 154: 4865-73, 1995. PMID: 7722336
- Nishimori I, Miyaji E, Morimoto K, Nagao K, Kamada M, Onishi S. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 54: 274-81, 2005. PMID: 15647194
- Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 118: 573-81, 2000. PMID: 10702209
- Okazaki K. Autoimmune pancreatitis: etiology, pathogenesis, clinical findings and treatment. The Japanese experience. JOP 6(1 Suppl): 89-96, 2005. PMID: 15650291
- Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 46:277-88, 2011. PMID: 21452084
- Okazaki K, Uchida K, Ikeura T, Takaoka M.Current concept and diagnosis of IgG4-related disease in the hepato-bilio-pancreatic system. J Gastroenterol. 48:303-14, 2013. PMID: 23417598
- Robinson D S, Larche M, Durham S R. Tregs and allergic disease. J Clin Invest 114: 1389-97, 2004. PMID: 15545986
- Roitt I. Antibodies. In: Roitt’s essential immunology. 9th ed. edited by Roitt I. London, Blackwell Science, 1997.
- Sarles H, Sarles J C, Muratore R.Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis 6: 688-98, 1961. PMID: 13746542
- Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 40:352-8, 2011. PMID: 21412117
- Taguchi M, Kihara Y, Nagashio Y, Yamamoto M, Otsuki M, Harada M. Decreased production of immunoglobulin M and A in autoimmune pancreatitis. J Gastroenterol 44:1133-9, 2009. PMID: 19626266
- Takizawa S, Endo T, Wanjia X, Tanaka S, Takahashi M, Kobayashi T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem Biophys Res Commun 386: 192-6, 2009. PMID: 19520060
- Uchida K, Okazaki K, Konishi Y, Ohana M, Takakuwa H, Hajiro K, Chiba T.Clinical analysis of autoimmune-related pancreatitis. Am J Gastroenterol 95: 2788-94, 2000. PMID: 11051349
- Uchida K, Okazaki K, Nishi T, Uose S, Nakase H, Ohana M, Matsushima Y, Omori K, Chiba T. Experimental immune-mediated pancreatitis in neonatally thymectomized mice immunized with carbonic anhydrase II and lactoferrin. Lab Invest 82: 411-24, 2002. PMID: 11950899
- Uchiyama-Tanaka Y, Mori Y, Kimura T, Sonomura K, Umemura S, 23Kishimoto N, Nose A, Tokoro T, Kijima Y, Yamahara H, Nagata T, Masaki H, Umeda Y, Okazaki K, Iwasaka T. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis 43: e18-25, 2004. PMID: 14981637
- Ueno Y, Ishii M, Takahashi S, Igarashi T, Toyota T, LaRusso N F. Different susceptibility of mice to immune-mediated cholangitis induced by immunization with carbonic anhydrase II. Lab Invest 78: 629-37, 1998. PMID: 9605187
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Nakamura S, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H.Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 22:21-30, 2012. PMID: 22620057
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, Tsubota K, Yoshino T, Kawa S, Suzuki R, Takegami T, Tomosugi N, Kurose N, Ishigaki Y, Azumi A, Kojima M, Nakamura S, Inoue D. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 22:1-14, 2012. PMID: 21881964
- van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317: 1554-7, 2007. PMID: 17872445
- Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 40: 1561-8, 1995. PMID: 7628283


