Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2021.09
| Attachment | Size |
|---|---|
| 1.65 MB |
Protein synthesis plays a central role in provision of pancreatic digestive enzymes and the maintenance of the pancreas. Both the mRNA profile and relative content of newly synthesized proteins are dominated by digestive enzymes. In the mature pancreas, about 90% of protein synthesis has been estimated to be devoted to a mixture of about 20 digestive enzymes (204). Whether the acinar cell can regulate digestive enzyme synthesis independent of the synthesis of cellular structural proteins is unclear. Pancreatic protein synthesis is regulated to match digestive enzyme synthesis to dietary need. Although the identity of individual synthesized proteins and long-term regulation is primarily determined by transcriptional regulation, the short-term extent, or rate of protein synthesis, is regulated at the translational level (161), because it needs to be immediate, flexible and reversible (123).
Figure 1. Overview of the main physiological stimulators of pancreatic digestive enzyme synthesis. Meal feeding stimulates the Central Nervous System (CNS) which, in turn, stimulates digestive enzyme synthesis via the vagus and acetylcholine release in the pancreas. Meal feeding stimulates the release of CCK from the “I” cells in the duodenum, that will directly stimulate the exocrine pancreas and vagal afferents, and insulin from the b cells in the Islets of Langerhans, that can also contribute to the regulation of pancreatic enzyme synthesis. Finally, some dietary nutrients, such as amino acids, will directly stimulate pancreatic digestive enzyme synthesis. (Figure modified from reference (158)).
In general, the gastrointestinal tract, including the exocrine pancreas, atrophies in the absence of food and protein synthesis that occurs in response to food intake is required to maintain normal function. Individual dietary components, like protein and amino acids (158, 162), regulate pancreatic protein synthesis in mice and rats (77, 78). In humans, feeding increases both the rate of secretion and synthesis of digestive enzymes, although the rate of zymogen turnover remains fairly constant during feeding and fasting (130). Pancreatic protein synthesis is also regulated by hormones such as cholecystokinin (CCK) and insulin, as well as by neural stimulation, all of which are influenced by food intake (Figure 1).
This review highlights how dietary elements and hormones affect intracellular effectors, such as mTORC1 and intracellular calcium concentration, to regulate certain steps of the pancreatic protein translational machinery, at the initiation and elongation levels. When stimulatory conditions are present, pancreatic protein synthesis is mainly stimulated through the mTORC1 pathway, but when there is an overstimulation of the pancreas, or a dietary imbalance, there is an increase on intracellular calcium concentrations, and cell stress, that trigger a signal which inhibits protein synthesis. These mechanisms coincide with stress in the endoplasmic reticulum (ER stress), resulting in unfolded/misfolded protein products retained in the ER, and the stimulation of several cell survival and cell death mechanisms.
I. Protein Synthesis Associated Mechanisms and their Regulation
The acinar cell of the exocrine pancreas has the greatest rate of protein synthesis of any mammalian organ, and it has long been used as a cell model to study the protein synthesis mechanisms in mammalian cells (85). Cooley, et al. nicely reviewed the protein synthesis pathways to secretion in pancreatic acinar cells, and we refer the reader to their publication on the Pancreapedia (31), for more detailed information.
Briefly, in eukaryotic cells, the ribosome - composed of two subunits: large (60S) and small (40S) are the central elements of the protein synthesis machinery and translate the sequence of an mRNA into the amino acid sequence of a protein. To initiate the process, the two ribosomal subunits are recruited together, with the mRNA and tRNA, by different proteins and eukaryotic initiation factors (eIF) on the cytosolic site of the ER membrane, and start the addition of the first amino acid to the peptide chain. This complex continues the addition of amino acids to form a polypeptide, by a process called elongation, assisted by eukaryotic elongation factors (130), and terminate the process by the help of termination factors (eTF). The newly formed secretory proteins will be inserted into the ER lumen through channels formed by proteins called translocons and are subject to several modifications. These include chaperoning and folding mechanisms that will yield mature proteins that are ready to be directed to their final destination (122, 173). Proteins destined to remain in the cell are synthesized by cytoplasmic ribosomes.
Pancreatic acinar cells devote most of their protein synthesis capacity to synthesize, and secrete, digestive enzymes (204). These mechanisms are directed to match the need for digesting the amount and quality of the different dietary elements. Meal to meal regulation needs to be immediate, reversible and flexible, and this is achieved by post-transcriptional processes directed at the regulation of the mRNA translation into protein (161).
The ER is an exceptionally prominent organelle in the acinar cell and is a major site of protein synthesis and transport, protein folding, lipid and steroid synthesis, carbohydrate metabolism and calcium storage (173).
A. mRNA translation.
Translation of mRNA into protein can be divided into three phases: initiation, elongation and termination (80, 121). During initiation (Figure 2), methionyl-tRNA initiator tRNA and several initiation factors associate with the 40S ribosomal subunit to form the 43S preinitiation complex. This complex binds to mRNA and migrates to the correct AUG initiation codon followed by the attachment of the 60S ribosomal subunit. Key regulated initiation factors in this process are the guanine nucleotide exchange factor eIF2B which activates eIF2, and eIF4E which recognizes the 5’(m7G)-cap of the mRNA. eIF4E is present in cells largely bound to its binding protein (4E-BP1) and is released when 4E-BP1 is phosphorylated on multiple sites. eIF4E interacts with the scaffolding protein eIF4G and eIF4A to form the eIF4F complex. The binding of eIF4F to an m7G cap commits the translational apparatus to the translation of the mRNA, and the ribosome will seek the start codon to start translation. The activation of S6 kinase and the phosphorylation of the ribosomal protein S6 enhance the translation of a specific set of mRNAs. In the elongation process, amino acids from amino acyl-tRNAs are added to the growing peptide in the order dictated by the mRNA bound to the ribosome. The key regulatory molecule is elongation factor 2 (eEF2) which catalyzes the translocation of the peptidyl tRNA from the A-site to the P-site on the ribosome (Figure 3) (41). In the termination phase the completed protein is released from the ribosome (121) and translocated into the ER lumen to continue its maturation steps, involving many chaperones and folding processes.
Figure 2. Initiation of protein synthesis in mammalian cells. During initiation, the initiator Met-tRNAi binds to a free 40S ribosomal subunit in a reaction requiring GTP bound to eIF2 to form the 43S preinitiation complex. In the next step, the eukaryotic initiation factor 4E (eIF4E) recognizes the capped end of the mRNA and interacts with the scaffolding protein eIF4G for form the eIF4F complex. After this, the 43S preinitiation complex will join the eIF4F complex and the ribosome will seek the start codon to start the process. A GTPase-activating protein promotes the hydrolysis of the GTP bound to eIF2 and releases eIF2-GDP. Regeneration of active eIF2-GTP is mediated by the guanine nucleotide-exchange factor eIF2B. The dissociation of the initiation factors allows the addition of the 60S subunit of the ribosome to form the 80S initiation complex, which is competent to enter elongation.
Figure 3. Protein elongation steps. The first step of elongation is the binding of an aminoacyl-tRNA to a vacant ribosomal A-site base pairing with the mRNA on the A-site. This process requires elongation factor 1A. The second step is peptidyl transference; the carboxyl end of the polypeptide chain uncouples from the tRNA molecule in the P-site and joins the amino acid linked to the tRNA molecule in the A-site by a peptide bond. The third step, Translocation, is catalyzed by elongation factor 2 (eEF2), and involves the translocation of the peptidyl-tRNA in the A-site to the P-site as the ribosome moves exactly three nucleotides along the mRNA molecule. The elongation process will repeat this cycle of amino acid addition to elongate protein polypeptides (121, 167).
B. Post-translational steps and associated processes.
As mentioned above, a protein destined for secretion must undergo proper folding and modifications, with the aid of chaperones and folding enzymes such as protein disulfide isomerase (PDI) and the immunoglobulin heavy chain binding proten (BiP). These interactions occur after nascent protein synthesis and translocation into the ER lumen (173). Despite all these specific and sophisticated controlled mechanisms, a fraction of proteins does not achieve native/mature and functional form and are either misfolded or aggregated (76). At this point, these proteins can: 1) remain in the ER, or 2) enter the ER-associated degradation (ERAD) pathway mediated by the proteasome. These steps insure that misfolded proteins do not inadvertently enter the secretory pathway (150). Retention of misfolded proteins in the ER can induce ER stress (69, 148) and a coordinated adaptive program called the Unfolded Protein Response (UPR) (199, 206). The UPR activates specific mechanisms directed to retain balance and proper function of the ER and the cell by: (i) inhibiting protein synthesis, (83) upregulating protein folding by enhancing translation of ER chaperone and folding enzymes, and (iii) activating degradation pathways associated with the ER; the ERAD (93). If the balance is not restored it can lead to cell death or apoptosis (188); thus, achieving normal function is critical for cell’s survival.
Three ER-resident transmembrane proteins have been identified as proximal sensors of ER stress: the kinase and endoribonuclease inositol requiring element 1 (IRE1) (202), the PKR-like ER kinase (PERK) (73) that phosphorylates the a subunit of eukaryotic initiation factor 2 (eIF2a) on its Ser51 residue and inhibits translation initiation (73), and the basic leucine-zipper activating transcription factor 6 (ATF6) (222). Phosphorylation of eIF2a by PERK inhibits general protein translation but allows preferential translation of mRNAs encoding several short upstream open reading frames like the mRNA for the activating transcription factor 4 (ATF4) (70). IRE1α dimerization followed by autophosphorylation triggers its mRNase activity, which processes the mRNA encoding unspliced X box-binding protein 1 (XBP1u) to produce and active transcription factor, spliced (XBP1s), that controls the transcription of genes encoding roteins involved in protein folding (81). The activation of the UPR may lead either to cell survival, by triggering the synthesis of ER chaperone proteins such as BiP and protein disulfide isomerase (PDI), along with a decrease in general protein translation, or to cell demise. The latter, occurs through the activation of programmed cell death signals (81, 199).
There are several connections to activation of ER stress response pathways and pathological human conditions (131). A malfunction of the ER stress response caused by aging, genetic mutations, or environmental factors can result in various diseases such as diabetes, inflammation, cancer, pancreatitis (shown below), non-alcoholic fatty liver disease, and neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, and bipolar disorder (111, 190, 221). How ER stress response pathways play a role in these pathologies is an active area of research and various components of the stress response pathways are being investigated as potential therapeutic targets (131, 152).
C. Main intracellular regulators
Intracellular calcium concentration
Calcium is a widespread signaling molecule that can affect different processes including localization, function and association of proteins, either with other proteins, organelles or nucleic acids. Ca2+, in addition to its role as an intracellular mediator of cell-surface humoral interactions, may function prominently in the regulation of post-transcriptional protein synthesis in a variety of eukaryotic cell types (126, 173). Early studies hypothesized that the rate of protein synthesis could be modulated in intact cells by varying the concentration and subcellular distribution of intracellular calcium, and it was thought that it was controlled by free cytosolic calcium rather than the sequestered cation. However, Brostrom, and Brostrom, 1990 (16), proposed that maintenance of optimal rates of protein synthesis depends on the amount of calcium sequestered in the endoplasmic reticulum rather than free cytosolic calcium, and several other studies in different cell types, confirmed this hypothesis (27, 94, 125).
Maintenance of free versus bound Ca2+ balance in all cellular compartments is critical for many cellular functions. This balance can be achieved, in part, thanks to calcium-binding proteins located in the cytoplasm or in specific organelles, that retain or release Ca2+ as needed. Some of these proteins (e.g. calnexin, calreticulin BiP, PDI) are located in the ER, and in addition to binding to calcium, function as chaperones, and help with protein folding (196). Integration of Ca2+ signaling in the lumen of the cellular reticular network including the ER, in mitochondria, the nucleus and in the cytoplasm provide integrated mechanisms for responding to cellular stresses by activation of appropriate coping responses.
In response to signal transduction generated inositol trisphosphate, calcium is released to the cytoplasm as part of the signal transduction cascade (173, 223). Sequestered calcium can also be released experimentally by treating cells with calcium ionophores (A23187), or inhibitors of the microsomal calcium-dependent ATPase such as Thapsigargin (Tg). All of these treatments disrupt protein folding in the ER and inhibit translation initiation, through an ER stress mechanism that involves phosphorylation of eIF2α by PKR, and the inhibition of eIF2B activity (16, 142, 179, 199). It has also been hypothesized that perturbation of the translocon, rather than suppression of protein processing, initiates the signal emanating from the ER culminating in eIF2α phosphorylation and translational repression. Therefore, sequestered Ca2+ from the ER can be seen as moderating the rate of mRNA translation in many cell types, including pancreatic acinar cells.
The importance of maintaining Ca2+ homeostasis and appropriate adaptation to ER stress is underlined by the accumulating evidence that constant disturbing Ca2+ homeostasis and chronic ER stress could lead to neurodegenerative disorders (189), diabetes (60, 225), cardiac hypertrophy (29) or cancer (201).
The mTORC1 pathway
The mTORC1 pathway is essential for cells to maintain homeostasis, providing tight control between the synthesis and degradation of cellular components, and it is the allosteric target of the drug rapamycin, that has clinical uses in organ transplantation, cardiology and oncology (144). mTORC1 connects environmental cues (nutrient and growth factor availability, as well as stress) to metabolic processes in order to preserve cellular homeostasis. Under nutrient-rich conditions mTORC1 promotes cell growth by stimulating biosynthetic pathways, including synthesis of proteins, lipids and nucleotides, and by inhibiting cellular catabolism through repression of the autophagic pathway (144). mTOR is an evolutionary conserved serine/threonine protein kinase that belongs to the PI3K-related kinase (PI3K) superfamily. This atypical kinase is the basis of two structurally and functionally different complexes termed mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (61). In addition to the mTOR catalytic subunit, mTORC1 consists of regulatory-associated protein of mammalian target of rapamycin (Raptor) (a scaffold protein that is required for the correct subcellular localization of mTORC1) (68, 92), mammalian lethal with Sec13 protein 8 (mLST8; also known as GßL) (which associates with the catalytic domain of mTOR and stabilizes the kinase activation loop) (68, 92, 220), and the two inhibitory subunits proline-rich Akt substrate of 40 kDa (PRAS40) (195) and DEP domain containing mTOR-interacting protein (DEPTOR) (141). Both, DEPTOR and PRAS40, are inhibitory proteins; phosphorylation blocks this inhibition. PRAS40 represents an essential component for insulin activation of mTORC1. Raptor is essential to mTORC1 function and its genetic deletion leads to loss of activity (204). mTORC2 contains, among other components, mTOR, mLST8, DEPTOR, the regulatory subunits mSin1, Rictor (rapamycin-insensitive companion of mTOR) (110), and regulates cell survival and proliferation primarily by phosphorylating several members of the AGC(PKA/PKG/PKC) family of protein kinases (30, 53).
Several proteins have been identified as substrates for phosphorylation by mTORC1. The first ones were proteins implicated in the control of mRNA translation, and this process remains one whose control by mTORC1 is best understood (143). mTORC1 functions to activate several steps in mRNA translation (phosphorylates S6 kinases, the inhibitory eIF4E-binding proteins (4E-BPs), and the eIF4G). Its signaling, requires amino acids (the precursors for protein synthesis) to activate these translation steps. Leucine is the most effective single amino acid that can stimulate of mTORC1 signaling (227). Finally, it has also been described that mTORC1 positively regulates translation elongation (143) and the protein degradation process through the proteasome, to increase the intracellular pool of amino acids, which will influence the rate of new protein synthesis (227) (Figure 4).
Deregulation of mTORC1 signalling increases the risk for metabolic diseases, including type 2 diabetes (11), cancer and epilepsy (30). In the exocrine pancreas, stimulation of the mTORC1 pathway has been shown to be activated by several hormones and nutrients, and leads the stimulation of acinar cell protein synthesis/digestive enzymes (164) (Figure 4). mTORC1 activation and signaling in pancreas is usually demonstrated by phosphorylation of downstream mediators, ribosomal protein S6 and 4E-BP1.
Figure 4. Scheme of the major mechanisms that regulate translation initiation in pancreatic acinar cells. G protein-coupled receptors, including CCK, stimulate translation initiation in pancreatic acinar cells through the phosphatidylinositol 3-kinase (PI3K) pathway. PI3K is activated in response to the active receptor and is believed to stimulate mTORC1 through phosphorylation of Akt/PKB, which, in turn, phosphorylates mTORC1. The round red knobs denote regulatory phosphate groups. mTORC1 is responsible (at least in part) for phosphorylating the eIF4E binding protein 4E-BP1 that allows the release of the mRNA cap-binding protein eIF4E, which is required for the formation of the eIF4F complex, which also includes eIF4G (a scaffolding protein) and eIF4A (a RNA helicase) and is necessary for the global increase in translation. mTOR also phosphorylates directly or through another kinase (multiple arrows) S6K1 (p70S6k), which is responsible for phosphorylating ribosomal protein S6 (S6) and thereby increasing the translation of mRNAs with polypyrimidine tracts. These mechanisms have been shown to be activated by meal feeding. eIF2 is required for Met-tRNA binding to the small ribosomal subunit. Phosphorylation of eIF2B (a GDP/GTP exchange factor) by GSK-3 results in inhibition of its GDP/GTP exchange activity. GSK-3 is itself inactivated (grey arrow) when it is phosphorylated by Akt/PKB. Thus the inactivation (phosphorylation) of GSK-3 causes dephosphorylation of eIF2B, leading to its activation, which enhances translation initiation. The phosphorylation of the α-subunit of eIF2 on Ser51 by several stress-activated kinases can inhibit eIF2B activity. In the present study, meal feeding did not significantly alter eIF2B activity. In the figure, protein kinases are shown in blue, translation initiation factors in yellow, and scaffolding or structural proteins in purple. (Modified from reference (161)).
II. Experimental Analysis of Pancreatic Protein Synthesis
Pancreatic protein synthesis has been traditionally measured by administering isotopically labeled amino acids with subsequent measurement of the incorporation of label into newly synthesized protein. For animal studies this is usually a radioactive isotope, while human studies have most often used stable isotopes (130). Several animal studies have measured pancreatic protein synthesis after diet-induced stimulation (91, 106, 161), hormonal stimulation (insulin, CCK) (13, 97, 98, 107, 133, 139, 160, 162, 163, 170), or after acute pancreatitis (55, 90, 108, 116, 132, 153). Among these studies, there are some discrepancies on the outcome of the results, that can be due to the different ways of administering the labeled amino acid as a tracer, and because some were lacking an accurate assessment of the specific radioactivity of the precursor amino acid at the site of protein synthesis.
The primary assumption was that the experimental treatment does not alter the relationship between the labeling of the sampled pool and that of the aminoacyl-tRNA, the direct precursor of protein synthesis, on in vivo experiments, specially. For those studies the readily accessible compartment pools, such as the intracellular free amino acids or plasma pools, to estimate precursor labeling, were used. However, experimental conditions have the potential to alter precursor enrichment either by affecting the amount of labeled amino acid entering the cell or by affecting the contribution of unlabeled amino acids derived from protein degradation to the charging of aminoacyl-tRNA. In these cases, the problem of accurately determining precursor enrichment can be minimized by the flooding dose technique, originally described by Garlick et al. (204), and validated by Sweiry et al. in rat pancreas (186).
With the flooding dose technique, the labeled amino acid is injected, not as a tracer, but contained in a large (i.e., much larger than the endogenous free amino acid pool) bolus of unlabeled amino acid, making the specific activities in all free amino acid compartments more alike than if the labeled compound is given as a tracer dose (155). Thus the labeling of aminoacyl-tRNA is less likely to be affected by experimental manipulations. In addition, the large amount of amino acid injected ensures that the specific activity in the free pools remains almost constant for a period of time after injection (39). Although various amino acids have been used, L-[3H]-phenylalanine was chosen as a radioactive tracer for experiments in the pancreas, because phenylalanine transport across the basolateral membrane of the pancreatic acinar cells is not rate-limiting for protein synthesis, and because [3H] can be easily quantitated by liquid scintillation counting (LSC) (186). The flooding dose technique is advantageous not only because it is reliable, but also because it can be used in unrestrained and unanesthetized animals (39) and has been well validated in muscle (134), liver (115), and pancreas (159, 161, 162, 186).
For in vitro studies, using isolated acini or pancreatic lobules, protein synthesis is measured by the incorporation of radioactively labeled amino acids, such as [35S]-Methionine (13, 160), or [3H]-Leucine (97), where the incorporation of these amino acids is linear during the experimental time because their intracellular pool is large and constant, due to the flooding of the extracellular space with an excess of amino acid, and therefore, there is no change in their specific activities (155).
III. Stimulation of pancreatic protein synthesis
A. Diet
Long-term regulation
Exocrine pancreatic adaptation to dietary changes has been observed in a variety of species (15, 169). The content and secretion of the digestive enzymes, proteases, amylase and lipases change in proportion to the dietary content of their respective substrates, protein, carbohydrate and fat. Changes in content of specific enzymes take place over 5-7 days. Similar changes occur in the synthesis of specific digestive enzymes measured by the incorporation of radioactive amino acids (38, 183, 210). Changes in individual digestive enzymes occur at both the transcriptional and translational levels but the major effect results from changes in specific mRNA levels. Various hormones mediate many of these effects and in most cases their release is increased by the nutrients whose digestion they regulate.
Proteases: A high protein diet (60-80% casein) increases the content of multiple proteases and the mRNA levels of trypsinogen, chymotrypsinogen and proelastase in rodents (15, 56). There are, however, differential effects on different isoforms of enzymes such as trypsinogen and this increase is not mimicked by feeding the individual amino acids (67). This is consistent with a potential effect of dietary protein (but not amino acids) on CCK release and increasing its plasma levels. Feeding soybean trypsin inhibitor (SBTI), an indirect stimulant of endogenous CCK release, or infusing CCK, increased trypsinogen I and chymotrypsinogen B mRNA in rats (149). Other studies using isolated pancreatic lobules showed that synthesis of proteases following infusion of the CCK analog caerulein in vivo was greatly increased, compared to a small increase in translatable mRNA, suggesting a post-transcriptional regulation of their synthesis (182, 211).
Lipases: The content and synthesis of pancreatic triglyceride lipase increases in response to a high fat diet (around 40-70% of calories as triglycerides) (15), and this is accompanied by an increase in its mRNA (147, 209). Secretin has been proposed as the mediator of the effect of dietary lipids (145), because fatty acids can stimulate secretin release, and infusion of secretin to rats, in vivo, induced an increase in the relative synthesis of pancreatic lipase (15).
Carbohydrates: The amount of carbohydrate in the diet has been shown to have significant effects on both pancreatic amylase content and amylase mRNA (15, 169). The effects of carbohydrate are believed to be primarily mediated by insulin. In early studies with diabetic animals, amylase content, synthesis, and mRNA levels, fall dramatically, while lipase increases moderately (169, 215). Insulin administration restores the amylase synthesis, content and mRNA levels in diabetic rats. However, insulin administration to normal rats either decreases or does not change pancreatic amylase levels. A more direct role for glucose on amylase synthesis, in addition to its indirect effects through insulin release, has been suggested (165). In fact, a dietary response sequence in the promoter of the amylase Amy2.2 gene has been identified that mediates dietary adaptation and the effect of insulin (171). The role of insulin in the regulation of pancreatic protein and digestive enzymes synthesis will be discussed in more detail later, in this review.
Short-term regulation
Meal stimulation: Only a few studies have evaluated the immediate regulation of the pancreatic translational synthetic machinery after food intake. Some early studies showed that fasting reduces total protein synthesis in the pancreas, and refeeding stimulates it (24, 106, 204). In humans, feeding increases both, the rate of secretion and synthesis of digestive enzymes, although the rate of turnover of zymogens remains fairly constant during feeding and fasting (130).
In mice, acute food intake stimulates pancreatic protein synthesis (Figure 5A) and translational effectors, without increasing digestive enzyme mRNA levels (Figure 5B) (161). These results indicate that acute feeding exerts a direct regulation of the mRNA translational machinery and a cellular reserve of untranslated mRNA, without changes in transcription.
Figure 5. Acute feeding in mice stimulates pancreatic protein synthesis without an increase in the mRNA for the digestive enzymes. (A) Pancreatic protein synthesis analyzed by the flooding dose technique, measuring Phe incorporation into pancreatic protein. Groups: Fed, refers to animals fed ad libitum, with pancreas removed at 9–10 AM. Fasted, refers to animals fasted for 18 h, and refed, refers to animals fasted for 18 h and refed for 1, 2, 3, or 6 h. Values are expressed as nanomoles of incorporated phenylalanine per milligram of protein per 10 min, and are the means ± SE. *P < 0.05 vs. fed group; #P < 0.05 vs. fasted group. (B) Relative mRNA quantities of digestive enzymes and housekeeping genes in pancreas of animals fed ad libitum, fasted for 18 h, and refed for 2 h. Values are the means ± SE. *P < 0.05 vs. fed group, †P < 0.05 vs. fasted group. (From reference (161)).
The stimulation of total pancreatic protein synthesis in mice and rats, is associated with the stimulation of the protein kinase B (PKB/Akt)/mTORC1 pathway, and the phosphorylation of 4E-BP1 and ribosomal protein S6, downstream of mTORC1, as well as the formation of the eIF4F complex (Figure 6) (161).
Meal/food intake stimulation of pancreatic digestive enzymes triggers a series of stimulatory mechanism through dietary elements, hormones and neural interactions (Figure 1), that are not completely understood, but this review summarizes what it is known about the most important ones.
Protein and amino acid stimulation: Protein and amino acids (104) are essential components of both human and animal nutrition and are involved in the maintenance of general health and well-being (89). Amino acids are the building blocks of protein, cell structures, and tissues, and can also act as regulators of protein metabolism and many physiological processes (168). A long-term deficiency of the essential AAs can lead to protein-energy malnutrition, manifested in humans as marasmus, or kwashiorkor (212). The therapeutic effects of amino acid when administered to patients, specially to infants, for growth as well as to maintain metabolism, have also been shown (124). AA supplementation specially benefits patients with chronic pancreatitis and pancreatic cancer because they suffer from malnutrition (172). The branched-chain amino acids (BCAAs), leucine, isoleucine, and valine, in particular, are considered biological regulators (198), in part, because they stimulate insulin release, play an important role in energy homeostasis (226), and are involved in the stimulation of cell proliferation in certain types of cancer (218). On the other hand, it has also been shown that the deficiency of BCAAs in the diet improves metabolic health in mice (205) and humans (48).
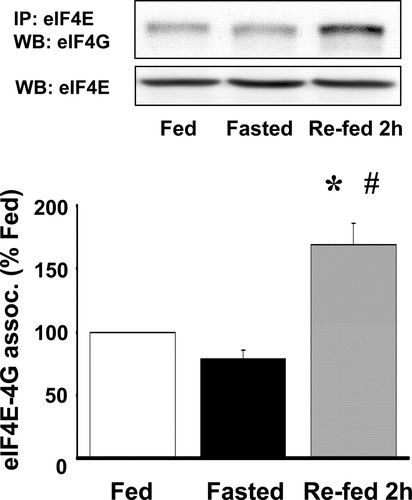
Figure 6. Effects of fasting and feeding on the eIF4F complex formation. eIF4F complex formation is measured as eIF4E and eIF4G coimmunoprecipitation. Data for coimmunoprecipitated eIF4E and eIF4G are expressed as percentage of fed group levels. Representative blots for eIF4G associated with eIF4E and total eIF4E are shown in the insets. *P < 0.05 vs. fed group, # P < 0.05 vs. fasted group. (From reference (161)).
The exocrine pancreas synthesizes and secretes between 6 and 20 g of digestive enzymes per day in humans (40, 45); it is very vulnerable to protein deficiency because it requires optimal nutrition for enzyme synthesis (40). The effects caused by dietary protein deficiency in the pancreas were shown by Crozier et al. (36), in a study done in mice that mimicked the kwashiorkor syndrome. Mice were fed a protein free diet for several days, resulting in pancreatic atrophy and the involvement of the mTORC1 pathway.
A high-protein diet (37, 77) and dietary amino acids (78) have been shown to stimulate pancreatic protein synthesis at the translation initiation level in mice and rats, and, at the same time, also stimulate pancreatic growth, because of their long-term treatment. Short-term effects of protein and AAs seem mainly to target the regulation of the protein synthetic machinery of pancreatic acinar cells through mTORC1 activation. BCAAs, especially leucine, can stimulate mTORC1 and the pancreatic protein synthesis machinery of rats, independently of CCK and insulin (162). Other studies also have shown the role of leucine in the exocrine pancreas of rats (78), pigs (128), and ruminants (23).
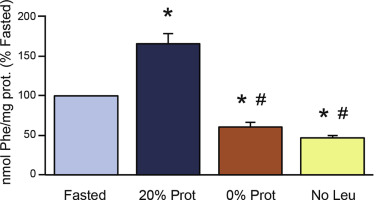
Figure 7. Effect of the different feeding treatments on total pancreatic protein synthesis. Mice were fasted for 16 hours, and refed with 3 experimental diets: control diet (20% Protein), protein-deficient diet (0% Prot), and leucine-deficient diet (No Leu). ∗P < .05 vs control fasted group. #P < .05 vs control refed group. (From reference (158)).
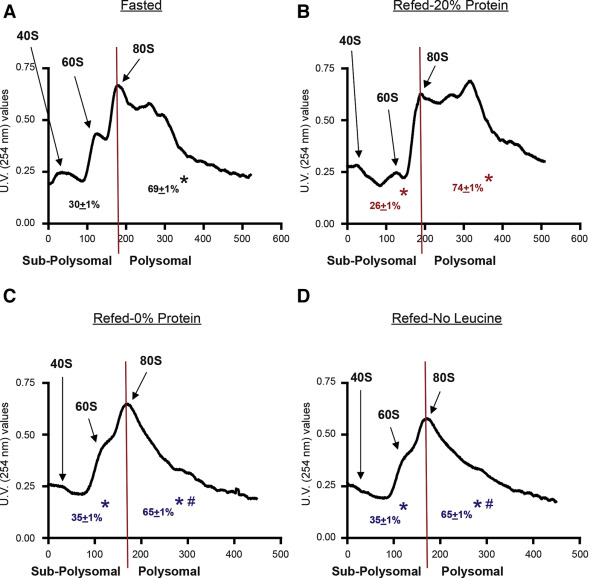
A short-term study in mice, demonstrated that diets lacking protein or amino acids inhibited pancreatic protein synthesis (measured by the flooding dose technique) (Figure 7) and the amount of ribosomes engaged in translation on a given moment, by polysomal fractionation (Figure 8) (158). Polysomal profiling analysis performed on pancreatic homogenates showed a significant increase in the polysomal fraction (i.e. more actively translated mRNAs into protein) in the control refed group (Figure 8B), compared to the control fasted group (Figure 8A). This was reflected in the analysis of the area under the curve of the polysomal fractions (Figure 8A and B). The groups lacking protein or leucine in the diet showed a very similar polysomal profile with a large reduction of the polysomal fraction, compared to the refed and fasted control groups (Figure 8C and D). The analysis of the area under the curve of these groups confirmed this reduction. At the same time, the analysis of the subpolysomal fraction indicated that refeeding with a 20% protein diet reduced the amount of free ribosomal subunits 40S and 60S, consistent with the increase in the 80S ribosomal unit and the polysomal fraction (Figure 8B). The lack of protein or leucine in the diet increased the subpolysomal peaks (area under the curve for both groups), indicating that there were more ribosomal subunits disengaged for translation (Figure 8C and D). These results confirm that total pancreatic protein synthesis was inhibited in the diets lacking protein or leucine, with a strong reduction of the mRNAs attached to the ribosomes for their translation into protein.
Figure 8. Effect of the 4 different experimental treatments on pancreatic polysomal profiles. Each profile shows the peaks for the 40S and 60S ribosomal subunits, as well as for the whole ribosome (80S) and the different polysomes, in the polysomal fraction for representative fractionations. The graphs also show the calculations of the area under the curve for the subpolysomal and the polysomal fractions. The experimental groups were as follows: (A) Fasted; (B) refed (20% Prot); (C) refed protein-deficient diet (0% Prot); and (D) refed leucine-deficient diet (No Leu). ∗P < .05 vs control group. #P < .05 vs control refed group. (From reference (158)).
This inhibition was accomplished through different intracellular mechanisms: the protein free diet inhibited pancreatic protein synthesis through a partial inhibition of the mTORC1 pathway, and of the eIF2B activity; the leucine free diet inhibition was caused by the inhibition of the eIF2B activity and activation of the stress signals leading to the GCN2 (General Control Nonderepressible 2) kinase activation and eIF2α phosphorylation, induced by the amino acid imbalance in the system and in the pancreas (158). This study shows that dietary AAs are important regulators of postprandial digestive enzyme synthesis, and their deficiency could induce pancreatic insufficiency and malnutrition.
Amino acids regulate mRNA translation in the pancreas through multiple mechanisms. One mechanism involves activation of mTOR that controls several downstream effectors, including RNA polymerase I, S6K1, 4E-BP1, and the elongation factor 2 kinase (eEF2K) (inactive when phosphorylated) (158). Amino acids, then, stimulate dephosphorylation (activation) of eEF2 that may also prevent activation of GCN2 by enhancing the removal of deacylated tRNA from the P-site on the ribosome (Figure 3); a potential activator of GCN2, or EIF2AK4 (eIF2α kinase 4). GCN2 is induced during amino acid deprivation by a mechanism that involves uncharged tRNA binding to a regulatory region homologous with HisRS (histidyl-tRNA synthetase) enzymes (207, 208). Phosphorylation of eIF2 impedes recycling of eIF2 to its active GTP-bound form (Figure 2), and the reduction in the levels of eIF2–GTP reduces global translation, allowing cells to conserve resources and to initiate a reconfiguration of gene expression to effectively manage stress conditions. A study using GCN2 deficient mice indicated an important effect of leucine deficiency in the translational machinery of the liver (2). Preventing activation of GCN2, amino acids preserve eIF2B activity, which promotes translation of all mRNAs, i.e. global protein synthesis is enhanced.
BCAAs, specifically leucine, have been shown to stimulate pancreatic protein synthesis in rats (162), dairy goats (22), and calves (64). Our study demonstrated that BCAAs stimulate the activation of mTORC1, analyzed by the phosphorylation of 4EBP1 and S6K (Figure 9A and B) and the formation of the eIF4F complex, independently of the hormones cholecystokinin (CCK) (Figure 9C) and insulin (162).
Figure 9. Effect of different amino acids on the activation of mTORC1 in vivo (A and B), and (C) effects of leucine on eIF4F complex formation in CCK/wild-type and CCK/KO mice. (A) 4E-BP1 phosphorylation, and (B) S6K phosphorylation on Thr-389, in rats 30 min after oral administration. (A) 4E-BP1 phosphorylation is expressed as the percentage of the protein present in the γ form. Inset shows representative immunoblot. The most highly phosphorylated γ-form exhibits the slowest electrophoretic mobility and does not bind eIF4E. (B) S6K phosphorylation is expressed as arbitrary units (AU). Inset shows a representative immunoblot for phosphorylated (p-S6K) and total S6K. Means not sharing a letter differ, P < 0.05. (C) eIF4F complex formation is expressed as a percentage of controls. Inset: representative immunoblot for eIF4G and eIF4E. Means not sharing a letter differ, P < 0.05. (From reference (162)).
Thus, dietary amino acids, as well as a mixed meal can stimulate the synthesis of digestive enzymes through the translational machinery by activating the mTORC1 pathway (Figures 5-9) (78, 158, 161, 162).
B. Cholecystokinin (CCK)
Regulation of translation initiation
As mentioned above, feeding stimulates pancreatic digestive enzyme synthesis at the translational level, and, in addition to dietary components, this process is also mediated by hormones such as CCK and insulin, and neurotransmitters such as acetylcholine (Figure 1). There are no conclusive published studies about the role of post-prandial CCK in the stimulation of pancreatic digestive enzyme synthesis, but unpublished results from our laboratory, using CCK deficient mice (105) in vivo, demonstrate that CCK is, in part, necessary for the stimulation of pancreatic digestive enzymes synthesis after feeding a complete and balanced diet, as well as for the stimulation of the mTORC1 pathway.
On the other hand, there are multiple studies showing the involvement of CCK in the stimulation of pancreatic acinar cell protein synthesis, and translational mechanisms, in vivo (14), and in vitro (12, 13, 97, 160).
In vivo : Bragado et al. (14) demonstrated that CCK, either administered by intraperitoneal injection to rats, or by stimulating endogenous CCK release by intragastric administration of a trypsin inhibitor, induced a time- and dose-dependent phosphorylation of pancreatic eIF4E and its binding protein 4EBP1 (or PHAS-I), as well as the formation of the eIF4F complex (Figures 2 and 4). These events occurred over a range of CCK doses from 0.2 to 5 μg/kg. The acute effects of endogenous CCK, released after gavaging a synthetic trypsin inhibitor, camostat (100 mg/kg), were similar. Thus, both exogenous and endogenous CCK activate translational initiation factors in vivo. The activation of translational machinery necessary for initiation of protein synthesis likely contributes to the normal postprandial synthesis of pancreatic digestive enzymes.
In vitro : CCK has a biphasic effect on enzyme secretion being stimulatory at physiological concentrations and inhibitory at higher concentrations, and follows the same biphasic pattern stimulating protein synthesis (12, 13, 160, 163) (Figure 10). Thus, CCK increases the rate of translation initiation (12, 13, 160, 163) and elongation processes (167) at physiological concentrations. That this acute stimulation of protein synthesis is at the translational level is shown by the fact that it occurs without a change in mRNA levels and in the presence of actinomycin D (107, 133). In these studies, the stimulation of protein synthesis occurred within 30 min and showed additivity between insulin and CCK. Increased synthesis of both digestive enzymes and structural proteins were observed, although differences between individual proteins suggested nonparallel translational effects (133).
Figure 10. Effect of CCK octapeptide on l-[35S]methionine incorporation into acinar protein. Rat pancreatic acini were incubated for 45 min with varying concentrations of CCK octapeptide before the addition of 2 μCi/ml of the labeled amino acid for 15 min. Incorporation was measured into TCA precipitable protein. *P < 0.05 vs. control (basal group). (From reference (160)).
Additionally, CCK or its analogue caerulein activate several regulators of translation, like the S6 kinase (S6K) (12, 159), the phosphorylation of eIF4E (14, 163), activate the formation of the eIF4F complex by stimulating the release of eIF4E from its binding protein 4E-BP1, and increase the association of eIF4E with eIF4G (14, 163). The activation of S6K, the formation of the eIF4F complex and the activation of the elongation processes and eEF2 appear to be regulated through a rapamycin - sensitive pathway and to be downstream of PI3K (12, 13, 167) (Figure 4).
Bragado et al. (12) also showed that the expression and activity of p70s6k-p85s6k (S6K) was biphasic after CCK stimulation. Carbachol and bombesin, but not vasoactive intestinal peptide, also activated S6K. This study concludes that the S6K stimulation by CCK is not mediated by PKC or mobilization of intracellular calcium but by PI3K. At the same time, it was shown that S6K is not involved in the secretion of digestive enzymes induced by CCK (12) (Figure 4).
Other stimulants, besides CCK, stimulate total protein synthesis in pancreatic acinar cells at their physiological concentrations. Carbachol, insulin and bombesin, in multiple species and preparations, all stimulate the synthesis of total protein, trypsinogen, chymotrypsinogen, lipase, and amylase by isolated acini (97, 98, 107).
Regulation of translation elongation
Stimulatory doses of cholecystokinin (CCK), bombesin, and carbachol, increased elongation rates (measured as ribosomal half-transit time) in pancreatic acini in vitro (Figure 11A). At the same time, these secretagogues reduced elongation factor 2 (eEF2) phosphorylation (the main factor known to regulate elongation), and increased the phosphorylation of the eEF2 kinase (Figure 11B and C). The mTOR inhibitor rapamycin reversed the dephosphorylation of eEF2 induced by CCK, as did treatment with the p38 MAPK inhibitor SB202190, the MEK inhibitor PD98059, and the phosphatase inhibitor calyculin A. Neither rapamycin, SB202190, PD98059 nor calyculin A had an effect on CCK mediated eEF2 kinase phosphorylation. Therefore, translation elongation in pancreatic acinar cells is likely regulated by eEF2 through the mTOR, p38, and MEK pathways, and modulated through PP2A (167).
Figure 11. Effects of several secretagogues on key elongation steps. (A)Average of the ribosomal half-transit time values ± SE, obtained after calculating the separation (in time) between the PMS (Post-Mitochondrial Supernatant) and PRS (Post-Ribosomal Supernatant) for each group. *p < 0:05 vs basal.(B) and (C) Effect of stimulatory doses of CCK, bombesin (BBS), carbachol (CCh) and the vasoactive intestinal peptide (VIP) on eEF2 (B) and eEF2K (C) phosphorylation. Acini were incubated for 60 min with CCK at 100 pM, BBS at 10 nM, CCh at 10 lM, and VIP at 100 nM. Results are expressed as a percentage of basal levels. *p < 0:05 vs basal. Blots are representative of phosphorylated and total eEF2 and eEF2K levels.(From reference (167)).
C. Insulin
The pancreas is composed of two separate organ systems; the exocrine pancreas, responsible for synthesizing the enzymes that will enter the intestine and participate in digestion, and the endocrine pancreas, composed of Islets of Langerhans, responsible for synthesizing insulin and other hormones that enter the blood and regulate glucose levels and metabolism. The physical location of both systems in the same organ and the common vascular supply, including a portal blood system by which venous blood from islets can perfuse the exocrine tissue, seems likely to have a purpose. In fact, the exocrine pancreas and the pancreatic islets have a multitude of complex anatomical and functional interrelations that can affect one another (6, 216). Among these, pancreatitis can induce diabetes, by damaging the endocrine cells of the islets (46, 57), and diabetes is correlated with exocrine pancreas insufficiency (75, 120). This indicates a role for insulin in the regulation of digestive enzyme synthesis and/or secretion.
For more detailed information on insulin secretion and action on pancreatic exocrine function, the reader is invited to check the reviews by Mann et al. (117), and by Sans et al. (157) on the Pancreapedia.
Briefly, insulin is known to stimulate protein synthesis by translational effects in many tissues (86). Early studies described the presence of insulin receptors in pancreatic acinar cells (100), and showed that insulin was involved in the incorporation of radioactive amino acids into total protein or specific enzymes of normal and diabetic rodents in vivo (34, 44, 101). Most of the studies showed a positive effect of insulin, but due to technical differences between studies, the results are difficult to interpret. When protein synthesis in mice was quantitated by autoradiography, there was more incorporation into peri-insular acinar cells than into tele-insular acinar cells (101), and this effect was lost after inducing diabetes with streptozotocin; implying that insulin had an effect on the peri-insular acinar cells. One study, with a different methodological approach, increased insulin secretion by treatment with sulfonylurea (a drug that stimulates insulin release) or by glucose infusion. Both treatments increased protein synthesis by 25 – 40%. When Zucker fatty rats were studied as a model of insulin resistance, overall pancreatic protein synthesis was reduced by nearly 50% (192). There was considerable difference in synthesis between different digestive enzymes separated by 2-D-gel electrophoresis. A morphological study of prolonged diabetes in rats showed gross abnormality in the acinar cell secretory pathway 28 days after STZ treatment that was partially reversed by insulin administration (219). However, shorter studies have not shown significant structural alterations 1 week after STZ other than the appearance of cytoplasmic lipid droplets (98).
More recent studies have been able to overcome some of the methodological issues by measuring the effects of insulin over short times to prevent changes in mRNA and under conditions where the precursor pool is large and constant (154, 155). In vitro studies have used isolated pancreatic acini under dilute labeling conditions to keep the precursor pool constant. Insulin stimulates the incorporation of multiple different amino acids (leucine, methionine, phenylalanine) into protein, in isolated acini from diabetic rats (97, 98, 107, 133). Similarly, insulin increased methionine incorporation into total protein and immunoprecipitated amylase in rat pancreas-derived AR42J cells (129). The effect of insulin on both cell types occurred over concentrations from 30 pM to 100 nM and was mediated by the insulin receptor (98, 133). Insulin was also shown to have nonparallel effects on different digestive enzymes and structural proteins such as myosin and LDH (133).
Although the mechanism of insulin signaling is well studied in a number of target tissues, especially liver, fat and muscle, only a few studies have been carried out in the exocrine pancreas. The main signaling pathway regulating protein synthesis is the mTORC1 pathway and this pathway has been documented in pancreatic acinar cells primarily in mediating the actions of CCK and acetylcholine to stimulate digestive enzyme synthesis (164, 213). Insulin has been shown to be involved in protein synthesis in several studies (13, 98, 107), and has nonparallel effects on translation of specific proteins (133). It also activates S6K and S6 phosphorylation downstream of TORC1 in rat and mouse acini (20, 184). Akt is upstream of mTORC1 and insulin activates the phosphorylation of Akt on S473 and T308 in rat acini (5). Bragado et al. (13) showed that insulin stimulates protein synthesis in pancreatic acinar cells in vitro, through the PI3K/Akt/mTPRC1 stimulated mechanisms (Figure 4). However, almost all of these studies were dependent on first inducing diabetes with streptozotocin to reduce endogenous levels of insulin, and diabetes effects could confound some of the results.
A recent study from our laboratory (to be published shortly) carried out in genetically modified mice in vivo, shows, for the first time, that insulin signaling through its receptor in pancreatic acinar cells, stimulates the synthesis of digestive enzymes after a balanced meal feeding (156). We developed a mouse model with a conditional insulin receptor (IR) KO in the pancreatic acinar cells: the pancreatic acinar cell insulin receptor knock out (PACIRKO) mice. It was obtained by crossing IRlox/lox mice with tamoxifen regulated Ela-Cre mice. The lack of insulin signaling to pancreatic acinar cells induced a 40% reduction in pancreas weight of PACIRKO mice, as well as total pancreatic protein and digestive enzymes content. The Akt/mTORC1 pathway activity was significantly reduced in both PACIRKO and diabetic mice, compared to normal control mice. Total pancreatic fluid secretion was not changed, but protein concentration and content of several digestive enzymes was reduced in the PACIRKO mice, compared to diabetic models. The specific deletion of the insulin receptor gene induced a decrease of pancreatic digestive enzymes content, an increase in the size of pancreatic acinar cell lumen, as well as apoptosis and activation of regeneration mechanisms in the pancreas with time. This study demonstrated that insulin directly regulates the synthesis of digestive enzymes in a post-prandial situation, as well as the short- and long-term exocrine pancreatic homeostasis.
This effect of insulin stimulating the synthesis of pancreatic digestive enzymes has clinical implications related to Type 1 diabetes, where insulin is reduced. A significant number of patients with diabetes suffer exocrine pancreatic abnormalities especially pancreatic exocrine insufficiency due to a reduction of the digestive enzymes, which occurs in about 50% of patients with type-1 diabetes (74). The decrease in pancreatic function correlates with the duration of both, type-1 and 2 diabetes (49, 104). In other studies with type 1 diabetes patients, atrophy of exocrine tissue and pancreatic fibrosis has been reported (50, 54, 104, 127). This pancreatic insufficiency during diabetes can affect physical development and metabolism in children and adolescent diabetic patients (51, 197).
Exocrine pancreatic abnormality has also been seen in animal models of diabetes (4, 88). Guinea pigs with spontaneous diabetes show fatty degeneration of pancreatic acinar cells and decreased digestive enzymes and bicarbonate secretion (3). Streptozotocin-treated diabetic rats show altered digestive enzyme gene expression, and reduced amylase secretion (99, 175, 177), without a decrease in the amount of fluid secreted (176). In isolated pancreatic acini, in vitro, there is a reduction of amylase secretion after STZ-induced diabetes (66, 135, 176, 217) due to reduced amylase pancreatic content (99, 177). More clinically relevant, mouse models of Type 1 diabetes like NOD mice or the Ins2Akita mouse model with insulin misfolding, also show alterations in the exocrine pancreas (119, 200).
From these studies it can be concluded that, in both, animals and humans, insulin (besides modulating the action of other hormones (CCK) or stimulators (ACh) (135, 176)) is necessary for the synthesis of digestive enzymes, and that these functions are inhibited in T1D.
IV. Intracellular Calcium Levels Affecting Pancreatic Protein Synthesis
CCK, and other G protein stimulating pancreatic secretagogues, inhibit pancreatic acinar protein synthesis at concentrations that hyperstimulate their receptors (160, 163). All of them have a common intracellular mechanism, increasing intracellular calcium to high concentrations (223, 224). High intracellular calcium levels have been shown to inhibit protein synthesis in other cell types (206), by ER stress and activation of the UPR (174). On the other hand, it has also been shown that removing calcium from the media, or depleting calcium from inside the acinar cells, also inhibits pancreatic protein synthesis (140, 160); indicating a need for calcium in the regulation of protein synthesis in pancreatic acinar cells.
A. Calcium signaling is involved in the stimulation of pancreatic protein synthesis
Changes in physiological calcium levels inside pancreatic acinar cells act as a signaling mechanism, that affect the activity of calcium-regulated enzymes (223). Calcineurin, also known as protein phosphatase 2B (PP2B), is a serine/threonine protein phosphatase that is highly regulated by Ca2+-calmodulin (95). This ubiquitously expressed phosphatase controls Ca2+-dependent processes in all human tissues; it has been found in the highest concentrations in the brain, but it has also been detected in many other mammalian tissues (19, 151), including the pancreas (19, 58). It is believed to be relatively inactive in cells under basal conditions of low intracellular calcium but becomes active after stimulation with calcium-mobilizing agonists (95). Calcineurin is best known for driving the adaptive immune response by dephosphorylating the nuclear factor of the activated T-cells (NFAT) family of transcription factors. Therefore, calcineurin inhibitors, FK506 (tacrolimus), and cyclosporin A serve as immunosuppressants. FK506 and cyclosporin A (CsA) binding to their intracellular target proteins (12-kDa FK506 binding protein and cyclophilin A) inhibits calcineurin phosphatase activity (28). The use of FK506 and CsA has been used to implicate calcineurin in a number of cellular processes, including pancreatic endocrine (43) and exocrine secretion (42, 58). Moreover, study of the side effects of CsA and FK506 in organ transplant therapy has implicated calcineurin in the protein synthesis mechanisms of some tissues, including kidney and liver (21). However, in contrast to the apparent multitude of cellular substrates for the type 1 and 2A serine/threonine phosphatases, relatively few cellular targets of calcineurin have been described (35, 95).
High concentrations of CsA were found to inhibit amylase secretion (58, 203), but it is not clear whether FK506 inhibits pancreatic exocrine secretion (42, 203) or not (unpublished observations). Both immunosuppressants were found to block pancreatic growth in response to chronic elevation of CCK induced by feeding trypsin inhibitor to mice (191), indicating a role for calcineurin in pancreatic growth (see the review on pancreatic growth by Williams, in Pancreapedia (214). Since, protein synthesis is an obligatory requirement for growth of all cells (138), calcineurin could also be involved in the regulation of pancreatic protein synthesis.
Using FK506 and CsA we determined that calcineurin is involved in the CCK stimulation of pancreatic acinar cell protein synthesis and translational machinery (163), demonstrating the involvement of intracellular calcium in the stimulation of mRNA translation into protein. FK506 also inhibited protein synthesis stimulated by bombesin and carbachol (Figure 12A). FK506 did not significantly affect the activity of the initiation factor-2B, or the phosphorylation of the initiation factor-2α, ribosomal protein protein S6, or the mRNA cap binding protein eukaryotic initiation factor (eIF)4E. Instead, blockade of calcineurin with FK506 reduced the phosphorylation of the eIF4E binding protein, decrease the formation of the eIF4F complex (Figure 12B), and increased the phosphorylation of eukaryotic elongation factor 2 (Figure 12C).
Figure 12. (A) Effect of FK506 on pancreatic protein synthesis stimulated with different agonists. The incorporation of [35S]methionine was analyzed in basal, CCK-stimulated (100 pM), bombesin (BBS)-stimulated (10 nM), and carbachol (CCh)-stimulated (30 μM) acini, with or without 100 nM FK506. In each experiment, 35S incorporation was normalized to control. *P < 0.05 vs. basal group; #P < 0.05 vs. its corresponding control stimulated group (CCK 100 pM; BBS 10 nM; CCh 30 μM). (B) Effects of FK506 on the formation of eIF4F, and (C) on eEF2 phosphorylation. (B) Formation of the eIF4F complex, measured as coimmunoprecipitation of eIF4E and eIF4G. (C) Phosphorylation of eEF2 (Thr-56) in response to CCK in the absence or presence of 100 nM FK506. Insets: representative Western blots for eIF4G and total eIF4E (B), and for phosphorylated and total eEF2 (C). *P < 0.05 vs. basal group; #P < 0.05 vs. control CCK-stimulated group. (From reference (163)).
From these results, it can be concluded that calcineurin activity and intracellular calcium are required for pancreatic protein synthesis, and, that this action may be related effects on the formation of the mRNA cap binding complex (Figure 1) and the elongation processes (Figure 2) (163).
B. High intracellular calcium levels inhibit pancreatic protein synthesis
In vitro
The inhibition of acinar protein synthesis and polysome size by CCK and the inhibitors of the microsomal Ca2+ ATPase, Thapsigargin (Tg), and 2,5-di(tertbutyl)-hydroquinone (Bhq), occurs as a result of depletion of Ca2+ from the endoplasmic reticulum lumen, increasing intracellular cytoplasmic calcium (140). The combination of administering the intracellular Ca2+ chelator, BAPTA with Tg and Bhq depleted the pools of [Ca2+] without changing cytosolic [Ca2+] but with the same inhibitory effect on protein synthesis and polysome formation, suggesting that depletion of intracellular Ca2+ stores, without changes in cytosolic [Ca2+], decreases protein synthesis at translation initiation (140). It has also been demonstrated that removal of Ca2+ from the medium enhanced the inhibitory action of CCK on both protein synthesis and eIF2B activity as well as further increased eIF2α phosphorylation (160) (Figure 13).
Figure 13. Mechanism by which high concentrations of CCK, and induction of ER stress inhibit initiation of translation. eIF2B activity is inhibited at high doses of CCK by release of intracellular Ca2+ and depletion of the intracellular stores, most likely from the ER lumen. This activates a kinase (such as PERK) that phosphorylates eIF2α. The ionophore A23187 and Thapsigargin (the inhibitor of the microsomal Ca2+- ATPase), also inhibit eIF2B activity through depletion of Ca2+ stores and phosphorylation of eIF2α (165, 166).
In the same study, with rat acini, Carbachol (CCh), and the ionophores A-23187 and Tg, also inhibited eIF2B and protein synthesis, whereas bombesin and the CCK analog JMV-180, that do not cause depletion of intracellular calcium stores at high concentrations, were without effect (Figure 14) (160).
Previous studies have shown that eIF2B can be negatively regulated by glycogen synthase kinase-3 (GSK-3). However, GSK-3 activity, as assessed by its phosphorylation state, was inhibited at high concentrations of CCK, an effect that should have stimulated, rather than repressed, eIF2B activity. Therefore, CCK inhibits eIF2B activity only through eIF2α phosphorylation (Figure 13).
Figure 14. Effect of high doses of CCK octapeptide, carbachol (CCh), bombesin (BBS), and the CCK analog (JMV-180) on isolated rat acini. Effects on (A) protein synthesis (B), eIF2B activity, and (C) eIF2α phosphorylation. Acini were incubated for 60 min with CCK at 10 nM, CCh at 1 mM, and BBS and JMV-180 at 1 μM and l-[35S]methionine incorporation determined as well as eIF2B activity, and eIF2α phosphorylation. Results are expressed as a percentage of basal levels. *P < 0.05 vs. control (basal group). The blots in (C) are representative for eIF2α phosphorylation levels. (From reference (160)).
In vivo
Inhibition of pancreatic protein synthesis in vivo normally occurs during the development of caerulein-induced acute pancreatitis (AP) (141), but it has been shown that total pancreatic protein synthesis is inhibited in some studies, whereas in others is not affected. On a study done using minced rabbit pancreas samples, a decrease in protein synthesis was observed in response to stimulatory doses of CCK, and this was accompanied by a decrease in the number of polysomes (139). Most of the differences seen on the effects of CCK in vivo, ex vivo, or in vitro, could be explained by methodological differences on the way of administering and analyzing the incorporation of AAs into protein.
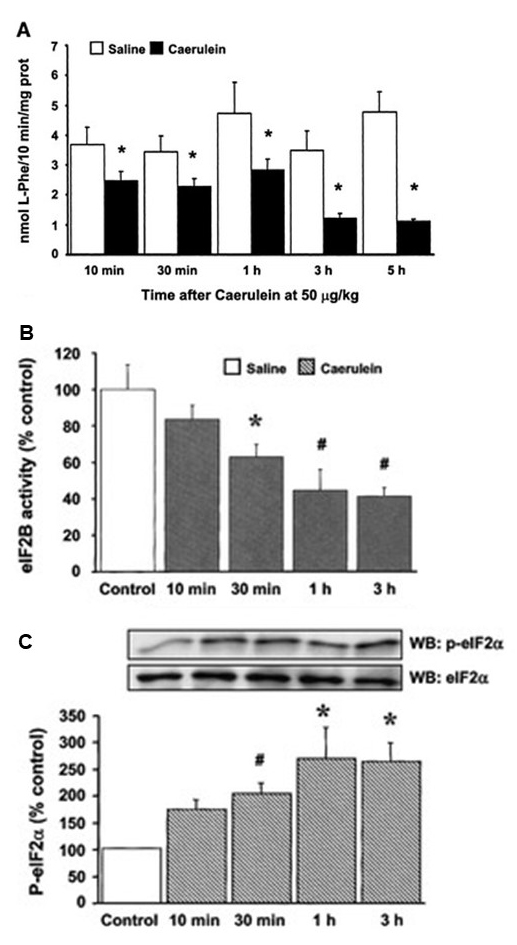
In a caerulein-induced acute pancreatitis (AP) study (159), pancreatic protein synthesis was already reduced 10 min after the initial caerulein administration and was further inhibited after three and five hourly injections (Figure 15A). Caerulein inhibited the two major regulatory points of translation initiation: the activity of the guanine nucleotide exchange factor eIF2B (with an increase of eIF2α phosphorylation) (Figure 15B and C), and the formation of the eIF4F complex, due, in part, to degradation of eIF4G (Figure 16). This inhibition was not accounted for by changes upstream (caerulein activated Akt), or downstream of mTORC1. Caerulein also decreased the phosphorylation of eEF2, implying that elongation was not inhibited during AP. Thus, the inhibition of pancreatic protein synthesis in this model of AP results from the inhibition of translation initiation due to increased eIF2a phosphorylation, reduction of eIF2B activity, and the inhibition of eIF4F complex formation (159). This inhibitory effect appears to be calcium-dependent, and suggests that pancreatic acinar cells adapt to this short–term stress (185) by inhibiting the synthesis of pancreatic digestive enzymes (139, 160). Although not yet fully studied, an ER resident kinase such as PERK (73) (Figure 13) is likely to mediate eIF2a phosphorylation and it has been shown to be important for the translational control and cell survival of pancreatic acinar cells (71, 72, 83). The inhibition of protein synthesis associated with high concentrations of CCK could therefore be an adaptive or protective mechanism in response to stress localized in the ER (159, 163).
Figure 15. Effect of caerulein-induced acute pancreatitis on pancreatic protein synthesis and on the first step of translation initiation (Figure 1), in mice. (A) l-[3H]phenylalanine incorporation into pancreatic protein in C57BL/6 mice. Values are expressed as nanomoles of incorporated phenylalanine per 10 min per milligram of protein, and are means and SE. *P < 0.05 vs. control (saline groups). (B) eukaryotic initiation factor (eIF)2B activity, and (C) eIF2α phosphorylation. The pancreas was removed 10 min, 30 min, and 1 h after a single-dose injection of caerulein at 50 μg/kg and 1 h after 3 hourly injections. The results are expressed as a percentage of pooled control levels. In C) the insets show representative blots for eIF2α phosphorylation levels and total eIF2α. For (B) and (C), data shown are means and SE. *P < 0.05, #P < 0.01 vs. pooled control group. (From reference (159)).
Figure 16. Effects of caerulein-induced acute pancreatitis on eIF4F complex formation, measured as eIF4E and eIF4G coimmunoprecipitation. (A) Data for coimmunoprecipitated eIF4G are expressed as percentage of pooled control levels and SE. Representative blots for eIF4G associated with eIF4E and total eIF4E are shown in the insets. #P < 0.01 vs. pooled control group. (B) representative Western blotting showing the degradation of total pancreatic eIF4G 30 min after caerulein injection. (From reference (159)).
Several early studies showed that pharmacological inhibition of protein synthesis during AP helped prevent acinar cell damage (59, 96). Later studies, on the contrary, have shown that a complete inhibition of pancreatic protein synthesis with cycloheximide during AP reduced pancreatic edema (1) but worsened the development of the disease and inhibited apoptotic pathways (87, 146) that have been proposed to be more beneficial than the necrotic ones in the severity of AP (7-9, 52, 118). Thus, a complete inhibition of pancreatic protein synthesis does not seem a likely event to happen during AP; rather, a selective inhibition of the synthesis of specific proteins would most likely account for the reduction seen in total protein synthesis during AP. Since synthesizing digestive enzymes in the exocrine pancreas is the main purpose of the gland under physiological situations, inhibition of this synthesis could likely happened during adverse situations such as AP, when secretion is also blocked, in an attempt to prevent or reduce more acinar cell damage.
Additionally, despite the fact that numerous studies have shown the possible interaction between lysosomal hydrolases and digestive enzyme zymogens in acinar cells in the development of AP (62, 103, 193, 194), whether lysosomal enzymes and pancreatic zymogens can be regulated at the translational level during AP has not been addressed.
B. Pancreatitis and the ER stress response
The events that regulate the severity of AP are, for the most part, unknown, and the exact mechanisms by which diverse etiological factors induce an attack are still unclear. The use of animal models of AP has permitted to advance in our understanding of the early cellular events that underlie the development of acute pancreatitis. However, there is still no understanding of a common triggering mechanism for the disease. These models share several common features including secretory blockade (108, 116, 153, 180), intracellular trypsin activation (82, 153, 181), high levels of digestive enzymes in blood, cytoplasmic vacuolization (116, 181), and activation of NFkB with later induction of an inflammatory response (25). Alterations in intracellular Ca2+ signaling, either by disturbances of calcium influx or by disturbances of calcium coming from the ER (17, 18, 47, 185), lead to the activation of multiple intracellular mechanisms that can involve the activation of ER stress, and it has been shown by us (92) and others (55, 90, 108, 116, 132, 159) that total protein synthesis is inhibited in AP.
ER stress signals are already active in normal pancreas in conditions that are important for the development of the gland (71), but these signals could likely be involved in the early stages of the development of the disease, to try to compensate any original imbalance. When the compensatory mechanisms are overworked, ER stress activates signaling cascades that ultimately lead to the activation of programmed cell death mechanisms (137).
Kubisch et al, showed, for the first time, that all major ER stress sensing and signaling mechanisms are present in pancreatic acini and are activated early in the arginine model of experimental acute pancreatitis (102). Arginine treatment caused an early activation of ER stress, as indicated by phosphorylation of PERK and its downstream target eIF2α, ATF6 translocation into the nucleus, and upregulation of BiP. XBP-1 splicing and CHOP expression were observed within 8 h. After 24 h, increased activation of the ER stress-related proapoptotic molecule caspase-12 was observed along with an increase in caspase-3 activity and TUNEL staining in exocrine acini. These results indicate that ER stress is an important early acinar cell event that could contribute to the development of acute pancreatitis and, with time, stimulate apoptosis in the arginine model.
Several studies directed at finding different physiologic and metabolic imbalances in the exocrine pancreas, such as oxidative stress, mitochondrial dysfunction and autophagy, deregulation of ATP generation and ER Acetyl-CoA (AT) availability, changes in lipid metabolism, inflammatory and cell death responses (10, 178), have demonstrated that these mechanisms often first manifest by abnormal cytosolic Ca2+ signaling. After abnormal calcium cytosolic levels, there is a multifaceted set of organelles and cellular interactions that trigger ER stress in pancreatic acinar cells, leading the way to different degrees of cell damage (84) and, ultimately, to acute (63, 65) and chronic (32) pancreatitis.
Other studies have demonstrated that mutations in some digestive enzymes, that can be hereditary, cause problems with their proper folding and trigger the ER stress response in the pancreas. This protein misfolding in the ER may also contribute to parenchymal damage by causing acinar cell death, and this may lead to chronic pancreatitis (79, 187)
A proteomics study on the RER of pancreatic acinar cells, from rat pancreases of control and two experimental models of AP (arginine- and caerulein-induced), revealed an increase of many ER proteins during AP, compared to the control group, but showed differences in the amount of several others between the two models of AP, possibly due to the different degree of cell damage caused by the two models, or to the different stages of the progression of cell damage (26). For instance, there was a clear reduction of some chaperone proteins, like BiP, in the arginine model, possibly due to the activation of apoptotic processes, the latest steps of the UPR mechanisms, when chaperoning for unfolded proteins is no longer needed.
Along these lines, Lugea and co-workers, suggest that the ER stress and UPR responses alone are not involved in the development of acute pancreatitis (113). They argue that to be considered as triggering factors of the development of AP and cause cell damage, these mechanisms, need to be combined with other environmental or genetic stressors. These authors study the effects of more clinically relevant causes of pancreatic injury, in experimental models. They show that alcohol abuse triggers a UPR response that attenuates the ER stress caused by alcohol to the pancreas, and suggest that early UPR mechanisms are beneficial for the wellbeing and survival of pancreatic acinar cells, because they are directed to compensate the original ER stress (113, 136). Lugea at al. demonstrate this hypothesis in another study (112), where the UPR was inhibited and cell death mechanisms were activated after applying, to AR42J cells, two well-known risk factors for clinical AP and chronic pancreatitis: cigarrete smoke and alcohol (33, 109).
To conclude, several insults that cause damage to pancreatic acinar cells, starting with a depletion of Ca2+ from ER stores, that induce an abnormal increase in cytosolic [Ca2+], and can inhibit protein and digestive enzymes synthesis and trigger an initial ER stress response. The ER stress response tries to compensate for these original imbalances, through the UPR, and when the cell situation can no longer be counteracted, the UPR stimulates cell death mechanisms through apoptosis. Some of these early ER stress mechanisms could likely be involved in the triggering events of AP, but it is also very likely that several insults need to be present to develop disease. A balancing regulation in response to ER-stress is essential for cell survival, and may act as a protective mechanism in the pancreas, as it does in other tissues. Because of that, it has also been proposed that the ER protein folding machinery and the UPR responses could be potential therapeutic targets to prevent and treat pancreatic diseases (114).
In the exocrine pancreas, ER stress mechanisms get activated when intracellular calcium concentrations increase to supramaximal levels (160), and during acute pancreatitis (102).
V. Acknowledgements
I want to express my gratitude to Prof. J. A. Williams for all his help, mentoring, scientific guidance, and collegiality during all these years working side by side. The research path is a hard road, and not always fairly compensated, but worth the ride, when you have such a mentor. Thank you, John, it wouldn’t have been possible without you!
VI. References
- Abe R, Shimosegawa T, Kimura K, Takasu A, Koizumi M, and Toyota T. Lipopolysaccharide-induced desensitization to pancreatic edema formation in rat cerulein pancreatitis. Pancreas 16: 539-544, 1998. PMID: 9598817.
- Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, and Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553-36561, 2004. PMID: 15213227.
- Balk MW, Lang CM, White WJ, and Munger BL. Exocrine pancreatic dysfunction in guinea pigs with diabetes mellitus. Lab Invest 32: 28-32, 1975. PMID: 1089836.
- Barreto SG, Carati CJ, Toouli J, and Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299: G10-G22, 2010. PMID: 20395539.
- Berna MJ, Tapia JA, Sancho V, Thill M, Pace A, Hoffmann KM, Gonzalez-Fernandez L, and Jensen RT. Gastrointestinal growth factors and hormones have divergent effects on Akt activation. Cell Signal 21: 622-638, 2009. PMID: 19166928.
- Bertelli E, Regoli M, Orazioli D, and Bendayan M. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia 44: 575-584, 2001. PMID: 11380075.
- Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med 8: 402-409, 2004. PMID: 15491516.
- Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189-G196, 2004. PMID: 14715516.
- Bhatia M, Wallig MA, Hofbauer B, Lee HS, Frossard JL, Steer ML, and Saluja AK. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun 246: 476-483, 1998. PMID: 9610387.
- Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D, Ruchala P, Whitelegge J, French SW, Wen L, Husain SZ, Gorelick FS, Hegyi P, Rakonczay Z, Jr., Gukovsky I, and Gukovskaya AS. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 154: 689-703, 2018. PMID: 29074451.
- Blandino-Rosano M, Barbaresso R, Jimenez-Palomares M, Bozadjieva N, Werneck-de-Castro JP, Hatanaka M, Mirmira RG, Sonenberg N, Liu M, Ruegg MA, Hall MN, and Bernal-Mizrachi E. Loss of mTORC1 signalling impairs beta-cell homeostasis and insulin processing. Nat Commun 8: 16014, 2017. PMID: 28699639.
- Bragado MJ, Groblewski GE, and Williams JA. p70s6k is activated by CCK in rat pancreatic acini. Am J Physiol Cell Physiol 273: C101-C109, 1997. PMID: PMID: 9252447.
- Bragado MJ, Groblewski GE, and Williams JA. Regulation of protein synthesis by cholecystokinin in rat pancreatic acini involves PHAS-I and the p70 S6 kinase pathway. Gastroenterology 115: 733-742, 1998. PMID: 9721171.
- Bragado MJ, Tashiro M, and Williams JA. Regulation of the initiation of pancreatic digestive enzyme protein synthesis by cholecystokinin in rat pancreas in vivo. Gastroenterology 119: 1731-1739, 2000. PMID: 11113094.
- Brannon PM. Adaptation of the exocrine pancreas to diet. Annu Rev Nutr 10: 85-105, 1990. PMID: 2200477.
- Brostrom CO, and Brostrom MA. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol 52: 577-590, 1990. PMID: 2184768.
- Bruce JI, and Elliott AC. Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase. Am J Physiol Cell Physiol 293: C938-C950, 2007. PMID: 17494627.
- Bruce JIE. Metabolic regulation of the PMCA: Role in cell death and survival. Cell Calcium 69: 28-36, 2018. PMID: 28625348.
- Burnham DB. Characterization of Ca2+-activated protein phosphatase activity in exocrine pancreas. Biochem J 231: 335-341, 1985. PMID: 2998347.
- Burnham DB, and Williams JA. Effects of carbachol, cholecystokinin, and insulin on protein phosphorylation in isolated pancreatic acini. J Biol Chem 257: 10523-10528, 1982. PMID: 7050109.
- Buss WC, and Stepanek J. Tissue specificity of translation inhibition in Sprague-Dawley rats following in vivo cyclosporin A. Int J Immunopharmacol 15: 775-782, 1993. PMID: 8407058.
- Cao Y, Liu K, Liu S, Guo L, Yao J, and Cai C. Leucine regulates the exocrine function in pancreatic tissue of dairy goats in vitro. Biomed Res Int 2019: 7521715, 2019. PMID: 31737677.
- Cao YC, Yang XJ, Guo L, Zheng C, Wang DD, Cai CJ, Liu SM, and Yao JH. Effects of dietary leucine and phenylalanine on pancreas development, enzyme activity, and relative gene expression in milk-fed Holstein dairy calves. J Dairy Sci 101: 4235-4244, 2018. PMID: 29477524.
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc 53: 211-354, 1978. PMID: 208670.
- Chen X, Ji B, Han B, Ernst SA, Simeone D, and Logsdon CD. NF-κB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 122: 448-457, 2002. PMID: 11832459.
- Chen X, Sans MD, Strahler JR, Karnovsky A, Ernst SA, Michailidis G, Andrews PC, and Williams JA. Quantitative organellar proteomics analysis of rough endoplasmic reticulum from normal and acute pancreatitis rat pancreas. J Proteome Res 9: 885-896, 2010. PMID: 19954227.
- Chin KV, Cade C, Brostrom CO, Galuska EM, and Brostrom MA. Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J Biol Chem 262: 16509-16514, 1987. PMID: 3680263.
- Clipstone NA, and Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357: 695-697, 1992. PMID: 1377362.
- Collins HE, Zhu-Mauldin X, Marchase RB, and Chatham JC. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol 305: H446-H458, 2013. PMID: 23792674.
- Condon KJ, and Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci 132: 2019. PMID: 31722960.
- Cooley MM, Jones EK, Gorelick FS, and Groblewski GE. Pancreatic acinar cell protein synthesis, intracellular transport, and export. In: Pancreapedia: Exocrine Pancreas Knowledge Base, 2020. DOI: 10.3998/panc.2020.15.
- Cooley MM, Thomas DDH, Deans K, Peng Y, Lugea A, Pandol SJ, Puglielli L, and Groblewski GE. Deficient endoplasmic reticulum acetyl-CoA import in pancreatic acinar cells leads to chronic pancreatitis. Cell Mol Gastroenterol Hepatol 11: 725-738, 2021. PMID: 33080365.
- Cote GA, Yadav D, Slivka A, Hawes RH, Anderson MA, Burton FR, Brand RE, Banks PA, Lewis MD, Disario JA, Gardner TB, Gelrud A, Amann ST, Baillie J, Money ME, O'Connell M, Whitcomb DC, Sherman S, and North American Pancreatitis Study G. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 9: 266-273; quiz e227, 2011. PMID: 21029787.
- Couture Y, Dunnigan J, and Morisset. Stimulation of pancreatic amylase secretion and protein synthesis by insulin. Scand J Gastroenterol 7: 257-263, 1972. PMID: 5034533.
- Creamer TP. Calcineurin. Cell Commun Signal 18: 137, 2020. PMID: 32859215.
- Crozier SJ, D'Alecy LG, Ernst SA, Ginsburg LE, and Williams JA. Molecular mechanisms of pancreatic dysfunction induced by protein malnutrition. Gastroenterology 137: 1093-1101, 1101 e1091-1093, 2009. PMID: 19427311.
- Crozier SJ, Sans MD, Wang JY, Lentz SI, Ernst SA, and Williams JA. CCK-independent mTORC1 activation during dietary protein-induced exocrine pancreas growth. Am J Physiol Gastrointest Liver Physiol 299: G1154-G1163, 2010. PMID: 20798356.
- Dagorn JC, and Lahaie RG. Dietary regulation of pancreatic protein synthesis. I. Rapid and specific modulation of enzyme synthesis by changes in dietary composition. Biochim Biophys Acta 654: 111-118, 1981. PMID: 6168287.
- Davis TA, Fiorotto ML, Nguyen HV, and Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab 277: E103-E109, 1999. PMID: 10409133.
- Descos L, Duclieu J, and Minaire Y. Exocrine pancreatic insufficiency and primitive malnutrition. Digestion 15: 90-95, 1977. PMID: 838182.
- Dever TE, Dinman JD, and Green R. Translation elongation and recoding in eukaryotes. Cold Spring Harb Perspect Biol 10: a032649, 2018. PMID: 29610120.
- Doi R, Inoue K, Chowdhury P, Kaji H, and Rayford PL. Structural and functional changes of exocrine pancreas induced by FK506 in rats. Gastroenterology 104: 1153-1164, 1993. PMID: 7681795.
- Donelan MJ, Morfini G, Julyan R, Sommers S, Hays L, Kajio H, Briaud I, Easom RA, Molkentin JD, Brady ST, and Rhodes CJ. Ca2+-dependent dephosphorylation of kinesin heavy chain on β-granules in pancreatic β-cells. Implications for regulated β-granule transport and insulin exocytosis. J Biol Chem 277: 24232-24242, 2002. PMID: 11978799.
- Duan RD, Wicker C, and Erlanson-Albertsson C. Effect of insulin administration on contents, secretion, and synthesis of pancreatic lipase and colipase in rats. Pancreas 6: 595-602, 1991. PMID: 1719525.
- El-Hodhod MA, Nassar MF, Hetta OA, and Gomaa SM. Pancreatic size in protein energy malnutrition: a predictor of nutritional recovery. Eur J Clin Nutr 59: 467-473, 2005. PMID: 15536474.
- Ewald N, and Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)--are we neglecting an important disease? Eur J Intern Med 24: 203-206, 2013. PMID: 23375619.
- Fanczal J, Pallagi P, Gorog M, Diszhazi G, Almassy J, Madacsy T, Varga A, Csernay-Biro P, Katona X, Toth E, Molnar R, Rakonczay Z, Jr., Hegyi P, and Maleth J. TRPM2-mediated extracellular Ca2+ entry promotes acinar cell necrosis in biliary acute pancreatitis. J Physiol 598: 1253-1270, 2020. PMID: 31917868.
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, and Lamming DW. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 16: 520-530, 2016. PMID: 27346343.
- Foulis AK, and Frier BM. Pancreatic endocrine-exocrine function in diabetes: an old alliance disturbed. Diabet Med 1: 263-266, 1984. PMID: 6242814.
- Foulis AK, and Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26: 456-461, 1984. PMID: 6381192.
- Frier BM, Saunders JH, Wormsley KG, and Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut 17: 685-691, 1976. PMID: 976808.
- Frossard JL, Rubbia-Brandt L, Wallig MA, Benathan M, Ott T, Morel P, Hadengue A, Suter S, Willecke K, and Chanson M. Severe acute pancreatitis and reduced acinar cell apoptosis in the exocrine pancreas of mice deficient for the Cx32 gene. Gastroenterology 124: 481-493, 2003. PMID: 12557153.
- Fu W, and Hall MN. Regulation of mTORC2 Signaling. Genes (Basel) 11: 2020. PMID: 32899613.
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619-633, 1965. PMID: 5318831.
- Gilliland L, and Steer ML. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physiol Gastrointest Liver Physiol 239: G418-G426, 1980. PMID: 6159794.
- Giorgi D, Renaud W, Bernard JP, and Dagorn JC. Regulation of proteolytic enzyme activities and mRNA concentrations in rat pancreas by food content. Biochem Biophys Res Commun 127: 937-942, 1985. PMID: 3885943.
- Gomez-Cerezo J, Garces MC, Codoceo R, Soto A, Arnalich F, Barbado J, and Vazquez JJ. Postprandial glucose-dependent insulinotropic polypeptide and insulin responses in patients with chronic pancreatitis with and without secondary diabetes. Regul Pept 67: 201-205, 1996. PMID: 8988521.
- Groblewski GE, Wagner AC, and Williams JA. Cyclosporin A inhibits Ca2+/calmodulin-dependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem 269: 15111-15117, 1994. PMID: 7515049.
- Gronroos JM, Aho HJ, Hietaranta AJ, and Nevalainen TJ. Early acinar cell changes in caerulein-induced interstitial acute pancreatitis in the rat. Exp Pathol 41: 21-30, 1991. PMID: 2022252.
- Guerrero-Hernandez A, and Verkhratsky A. Calcium signalling in diabetes. Cell Calcium 56: 297-301, 2014. PMID: 25217232.
- Guertin DA, and Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 12: 9-22, 2007. PMID: 17613433.
- Guillaumes S, Blanco I, Villanueva A, Sans MD, Clave P, Chabas A, Farre A, and Lluis F. Chloroquine stabilizes pancreatic lysosomes and improves survival of mice with diet-induced acute pancreatitis. Pancreas 14: 262-266, 1997. PMID: 9094156.
- Gukovskaya AS, Gorelick FS, Groblewski GE, Mareninova OA, Lugea A, Antonucci L, Waldron RT, Habtezion A, Karin M, Pandol SJ, and Gukovsky I. Recent insights into the pathogenic mechanism of pancreatitis: role of acinar cell organelle disorders. Pancreas 48: 459-470, 2019. PMDI: 30973461.
- Guo L, Yao JH, Zheng C, Tian HB, Liu YL, Liu SM, Cai CJ, Xu XR, and Cao YC. Leucine regulates α-amylase and trypsin synthesis in dairy calf pancreatic tissue in vitro via the mammalian target of rapamycin signalling pathway. Animal 13: 1899-1906, 2019. PMID: 30616697.
- Habtezion A, Gukovskaya AS, and Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology 156: 1941-1950, 2019. PMID: 30660726.
- Han J, and Liu YQ. Suppressed glucose metabolism in acinar cells might contribute to the development of exocrine pancreatic insufficiency in streptozotocin-induced diabetic mice. Metabolism 59: 1257-1267, 2010. PMID: 20051281.
- Hara H, Hashimoto N, Akatsuka N, and Kasai T. Induction of pancreatic trypsin by dietary amino acids in rats: four trypsinogen isozymes and cholecystokinin messenger RNA. J Nutr Biochem 11: 52-59, 2000. PMID: 15539343.
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, and Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177-189, 2002. PMID: 12150926.
- Harding HP, Calfon M, Urano F, Novoa I, and Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol 18: 575-599, 2002. PMID: 12142265.
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, and Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099-1108, 2000. PMID: 11106749.
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, and Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153-1163, 2001. PMID: 11430819.
- Harding HP, Zhang Y, Bertolotti A, Zeng H, and Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897-904, 2000. PMID: 10882126.
- Harding HP, Zhang Y, and Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271-274, 1999. PMID: 9930704.
- Hardt PD, and Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res 2011: 761950, 2011. PMID: 21822421.
- Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Jaeger C, Teichmann J, Bretzel RG, Hollenhorst M, and Kloer HU. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology 3: 395-402, 2003. PMID: 14526149.
- Hartl FU, and Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16: 574-581, 2009. PMID: 19491934.
- Hashi M, Yoshizawa F, Onozuka E, Ogata M, and Hara H. Adaptive changes in translation initiation activities for rat pancreatic protein synthesis with feeding of a high-protein diet. J Nutr Biochem 16: 507-512, 2005. PMID: 16043033.
- Hashimoto N, and Hara H. Dietary amino acids promote pancreatic protease synthesis at the translation stage in rats. J Nutr 133: 3052-3057, 2003. PMID: 14519783.
- Hegyi E, and Sahin-Toth M. Human CPA1 mutation causes digestive enzyme misfolding and chronic pancreatitis in mice. Gut 68: 301-312, 2019. PMID: 30045879.
- Hershey JWB, and Merrick WC. Pathway and mechanism of initiation of protein synthesis. In: Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press. p: 33-88, 2000. PMID: 30045879.
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89-102, 2012. PMID: 22251901.
- Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, and Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 275: G352-G362, 1998. PMID: 9688663.
- Iida K, Li Y, McGrath BC, Frank A, and Cavener DR. PERK eIF2α kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol 8: 38, 2007. PMID: 17727724.
- Ito M, Nakagawa H, Okada T, Miyazaki S, and Matsuo S. ER-stress caused by accumulated intracistanal granules activates autophagy through a different signal pathway from unfolded protein response in exocrine pancreas cells of rats exposed to fluoride. Arch Toxicol 83: 151-159, 2009. PMID: 18696052.
- Jamieson JD, and Palade GE. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol 50: 135-158, 1971. PMID: 4327462.
- Jefferson LS. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes 29: 487-496, 1980. PMID: 6991336.
- Kaiser AM, Saluja AK, Lu L, Yamanaka K, Yamaguchi Y, and Steer ML. Effects of cycloheximide on pancreatic endonuclease activity, apoptosis, and severity of acute pancreatitis. Am J Physiol Cell Physiol 271: C982-C993, 1996. PMID: 8843729.
- Kang JH, Na KJ, Mo IP, Chang D, and Yang MP. Juvenile diabetes mellitus accompanied by exocrine pancreatic insufficiency in a dog. J Vet Med Sci 70: 1337-1340, 2008. PMID: 19122401.
- Karau A, and Grayson I. Amino acids in human and animal nutrition. Adv Biochem Eng Biotechnol 143: 189-228, 2014. PMID: 24676880.
- Kern HF, Adler G, and Scheele GA. Structural and biochemical characterization of maximal and supramaximal hormonal stimulation of rat exocrine pancreas. Scand J Gastroenterol Suppl 112: 20-29, 1985. PMID: 3859914.
- Kern HF, Rausch U, and Scheele GA. Regulation of gene expression in pancreatic adaptation to nutritional substrates or hormones. Gut 28 Suppl: 89-94, 1987. PMID: 3319815.
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, and Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163-175, 2002. PMID: 12150925.
- Kim R, Emi M, Tanabe K, and Murakami S. Role of the unfolded protein response in cell death. Apoptosis 11: 5-13, 2006. PMID: 16374548.
- Kimball SR, and Jefferson LS. Regulation of protein synthesis by modulation of intracellular calcium in rat liver. Am J Physiol Endocrinol Metab 263: E958-E964, 1992. PMID: 1359794.
- Klee CB, Ren H, and Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273: 13367-13370, 1998. PMID: 9593662.
- Korbova L, Kohout J, Malis F, Balas V, Cizkova J, Marek J, and Cihak A. Inhibitory effect of various cytostatics and cycloheximide on acute experimental pancreatitis in rats. Gut 18: 913-918, 1977. PMID: 73498.
- Korc M, Bailey AC, and Williams JA. Regulation of protein synthesis in normal and diabetic rat pancreas by cholecystokinin. Am J Physiol Gastrointest Liver Physiol 241: G116-G121, 1981. PMID: 6791509.
- Korc M, Iwamoto Y, Sankaran H, Williams JA, and Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. I. Stimulation of protein synthesis. Am J Physiol Gastrointest Liver Physiol 240: G56-G62, 1981. PMID: 6161541.
- Korc M, Owerbach D, Quinto C, and Rutter WJ. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science 213: 351-353, 1981. PMID: 6166044.
- Korc M, Sankaran H, Wong KY, Williams JA, and Goldfine ID. Insulin receptors in isolated mouse pancreatic acini. Biochem Biophys Res Commun 84: 293-299, 1978. PMID: 363126.
- Kramer MF, and Poort C. The effect of insulin on amino acid incorporation into exocrine pancreatic cells of the rat. Horm Metab Res 7: 389-393, 1975. PMID: 1183917.
- Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, and Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 291: G238-G245, 2006. PMID: 16574987.
- Kukor Z, Mayerle J, Kruger B, Toth M, Steed PM, Halangk W, Lerch MM, and Sahin-Toth M. Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J Biol Chem 277: 21389-21396, 2002. PMID: 11932257.
- Laass MW, Henker J, Thamm K, Neumeister V, and Kuhlisch E. Exocrine pancreatic insufficiency and its consequences on physical development and metabolism in children and adolescents with type 1 diabetes mellitus. Eur J Pediatr 163: 681-682, 2004. PMID: 15300430.
- Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, and Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol Gastrointest Liver Physiol 276: G1302-G1309, 1999. PMID: 10330022.
- Lahaie RG. Dietary regulation of protein synthesis in the exocrine pancreas. J Pediatr Gastroenterol Nutr 3 Suppl 1: S43-50, 1984. PMID: 6209378.
- Lahaie RG. Translational control of protein synthesis in isolated pancreatic acini: role of CCK8, carbachol, and insulin. Pancreas 1: 403-410, 1986. PMID: 2436215.
- Lampel M, and Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 373: 97-117, 1977. PMID: 139754.
- Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB, and Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol 104: 2797-2805; quiz 2806, 2009. PMID: 19603011.
- Laplante M, and Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274-293, 2012. PMID: 22500797.
- Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, and Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 69: 927-947, 2018. PMID: 29940269.
- Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu C, French SW, Wilson JS, Apte MV, Waldron RT, and Pandol SJ. The combination of alcohol and cigarette smoke induces endoplasmic reticulum stress and cell death in pancreatic acinar cells. Gastroenterology 153: 1674-1686, 2017. PMID: 28847752.
- Lugea A, Waldron RT, and Pandol SJ. Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response. Pancreatology 15: S1-5, 2015. PMID: 25736240.
- Lukas J, Pospech J, Oppermann C, Hund C, Iwanov K, Pantoom S, Petters J, Frech M, Seemann S, Thiel FG, Modenbach JM, Bolsmann R, de Freitas Chama L, Kraatz F, El-Hage F, Gronbach M, Klein A, Muller R, Salloch S, Weiss FU, Simon P, Wagh P, Klemenz A, Kruger E, Mayerle J, Delcea M, Kragl U, Beller M, Rolfs A, Lerch MM, and Sendler M. Role of endoplasmic reticulum stress and protein misfolding in disorders of the liver and pancreas. Adv Med Sci 64: 315-323, 2019. PMID: 30978662.
- Lundholm K, Ternell M, Zachrisson H, Moldawer L, and Lindstrom L. Measurement of hepatic protein synthesis in unrestrained mice-evaluation of the 'flooding technique'. Acta Physiol Scand 141: 207-219, 1991. PMID: 2048407.
- Machovich R, Papp M, and Fodor I. Protein synthesis in acute pancreatitis. Biochem Med 3: 376-383, 1970. PMID: 5523414.
- Mann E, Sunni M, and Bellin MD. Secretion of insulin in response to diet and hormones. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc.2020.16.
- Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, and Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem 281: 3370-3381, 2006. PMID: 16339139.
- Meagher C, Tang Q, Fife BT, Bour-Jordan H, Wu J, Pardoux C, Bi M, Melli K, and Bluestone JA. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol 180: 7793-7803, 2008. PMID: 18523243.
- Meisterfeld R, Ehehalt F, Saeger HD, and Solimena M. Pancreatic disorders and diabetes mellitus. Exp Clin Endocrinol Diabetes 116 Suppl 1: S7-S12, 2008. PMID: 18777459.
- Merrick WC. The protein biosynthesis elongation cycle. In: Translational Control of Gene Expression Monograph. Cold Spring Harbor Laboratory Press. p: 89-125, 2000.
- Merrick WC, and Pavitt GD. Protein synthesis initiation in eukaryotic cells. In: Cold Spring Harb Perspect Biol. Cold Spring Harbor Laboratory Press. p: a033092, 2018.
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem 267: 6321-6330, 2000. PMID: 11029573.
- Millward DJ. Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. Br J Nutr 108 Suppl 2: S3-21, 2012. PMID: 23107542.
- Miyamoto S, Patel P, and Hershey JW. Changes in ribosomal binding activity of eIF3 correlate with increased translation rates during activation of T lymphocytes. J Biol Chem 280: 28251-28264, 2005. PMID: 15946946.
- Mockett BG, Guevremont D, Wutte M, Hulme SR, Williams JM, and Abraham WC. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci 31: 7380-7391, 2011. PMID: 21593322.
- Mohapatra S, Majumder S, Smyrk TC, Zhang L, Matveyenko A, Kudva YC, and Chari ST. Diabetes mellitus is associated with an exocrine pancreatopathy: conclusions from a review of literature. Pancreas 45: 1104-1110, 2016. PMID: 26918874.
- Morales A, Buenabad L, Castillo G, Vazquez L, Espinoza S, Htoo JK, and Cervantes M. Dietary levels of protein and free amino acids affect pancreatic proteases activities, amino acids transporters expression and serum amino acid concentrations in starter pigs. J Anim Physiol Anim Nutr (Berl) 101: 723-732, 2017. PMID: 27121753.
- Mossner J, Logsdon CD, Williams JA, and Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar AR42J cells. Diabetes 34: 891-897, 1985. PMID: 2411617.
- O'Keefe S J, Lee RB, Li J, Zhou W, Stoll B, and Dang Q. Trypsin and splanchnic protein turnover during feeding and fasting in human subjects. Am J Physiol Gastrointest Liver Physiol 290: G213-G221, 2006. PMID: 16123201.
- Oakes SA, and Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10: 173-194, 2015. PMID: 25387057.
- Ogden JM, Modlin IM, Gorelick FS, and Marks IN. Effect of buprenorphine on pancreatic enzyme synthesis and secretion in normal rats and rats with acute edematous pancreatitis. Dig Dis Sci 39: 2407-2415, 1994. PMID: 7525167.
- Okabayashi Y, Moessner J, Logsdon CD, Goldfine ID, and Williams JA. Insulin and other stimulants have nonparallel translational effects on protein synthesis. Diabetes 36: 1054-1060, 1987. PMID: 3301474.
- Otsuki M, Okabayashi Y, Ohki A, Suehiro I, and Baba S. Dual effects of hydrocortisone on exocrine rat pancreas. Gastroenterology 93: 1398-1403, 1987. PMID: 2445619.
- Otsuki M, and Williams JA. Effect of diabetes mellitus on the regulation of enzyme secretion by isolated rat pancreatic acini. J Clin Invest 70: 148-156, 1982. PMID: 6177717.
- Pandol SJ, Gorelick FS, Gerloff A, and Lugea A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis 28: 776-782, 2010. PMID: 21525762.
- Pandol SJ, Gorelick FS, and Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function - a hypothesis. Front Physiol 2: 8, 2011. PMID: 21483727.
- Pardee AB. G1 events and regulation of cell proliferation. Science 246: 603-608, 1989. PMID: 2683075.
- Perkins PS, and Pandol SJ. Cholecystokinin-induced changes in polysome structure regulate protein synthesis in pancreas. Biochim Biophys Acta 1136: 265-271, 1992. PMID: 1381613.
- Perkins PS, Park JH, and Pandol SJ. The role of calcium in the regulation of protein synthesis in the exocrine pancreas. Pancreas 14: 133-141, 1997. PMID: 9057185.
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, and Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873-886, 2009. PMID: 19446321.
- Prostko CR, Brostrom MA, and Brostrom CO. Reversible phosphorylation of eukaryotic initiation factor 2 alpha in response to endoplasmic reticular signaling. Mol Cell Biochem 127-128: 255-265, 1993. PMID: 7935356.
- Proud CG. mTORC1 signalling and mRNA translation. Biochem Soc Trans 37: 227-231, 2009. PMID: 19143637.
- Rabanal-Ruiz Y, Otten EG, and Korolchuk VI. mTORC1 as the main gateway to autophagy. Essays Biochem 61: 565-584, 2017. PMID: 29233869.
- Rausch U, Rudiger K, Vasiloudes P, Kern H, and Scheele G. Lipase synthesis in the rat pancreas is regulated by secretin. Pancreas 1: 522-528, 1986. PMID: 3645660.
- Resau JH, Marzella L, Jones RT, and Trump BF. What's new in in vitro studies of exocrine pancreatic cell injury? Pathol Res Pract 179: 576-588, 1985. PMID: 3889889.
- Ricketts J, and Brannon PM. Amount and type of dietary fat regulate pancreatic lipase gene expression in rats. J Nutr 124: 1166-1171, 1994. PMID: 8064366.
- Ron D, and Harding HP. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb Perspect Biol 4: 2012. PMID: 23209157.
- Rosewicz S, Dunbar Lewis L, Wang X-Y, Liddle RA, and Logsdon CD. Pancreatic digestive enzyme gene expression: effects of CCK and soybean trypsin inhibitor. Am J Physiol Gastrointest Liver Physiol 256: G733-G738, 1989. PMID: 2468294.
- Ruggiano A, Foresti O, and Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol 204: 869-879, 2014. PMID: 24637321.
- Rusnak F, and Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483-1521, 2000. PMID: 11015619.
- Ryno LM, Wiseman RL, and Kelly JW. Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases. Curr Opin Chem Biol 17: 346-352, 2013. PMID: 23647985.
- Saluja AK, Saito I, Saluja M, Houlihan MJ, Powers RE, Meldolesi J, and Steer M. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol Gastrointest Liver Physiol 249: G702-G710, 1985. PMID: 2417493.
- Sankaran H, Iwamoto Y, Korc M, Williams JA, and Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol Gastrointest Liver Physiol 240: G63-G68, 1981. PMID: 7006418.
- Sans MD. Measurement of pancreatic protein synthesis. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2010. DOI: 10.3998/panc.2010.16.
- Sans MD, Amin RK, Vogel NL, D'Alecy LG, Kahn RC, and Williams JA. Specific deletion of insulin receptors on pancreatic acinar cells defines the insulin-acinar axis: implications for pancreatic insufficiency in diabetes. Gastroenterology 140: A-233, 2011.
- Sans MD, Bruce JI, and Williams JA. Regulation of pancreatic exocrine function by islet hormones. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc.2020.01.
- Sans MD, Crozier SJ, Vogel NL, D'Alecy LG, and Williams JA. Dietary protein and amino acid deficiency inhibit pancreatic digestive enzyme mRNA translation by multiple mechanisms. Cell Mol Gastroenterol Hepatol 11: 99-115, 2021. PMID: 32735995.
- Sans MD, DiMagno MJ, D'Alecy LG, and Williams JA. Caerulein-induced acute pancreatitis inhibits protein synthesis through effects on eIF2B and eIF4F. Am J Physiol Gastrointest Liver Physiol 285: G517-G528, 2003. PMID: 12773302.
- Sans MD, Kimball SR, and Williams JA. Effect of CCK and intracellular calcium to regulate eIF2B and protein synthesis in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 282: G267-G276, 2002. PMID: 11804848.
- Sans MD, Lee SH, D'Alecy LG, and Williams JA. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol 287: G667-G675, 2004. PMID: 15117679.
- Sans MD, Tashiro M, Vogel NL, Kimball SR, D'Alecy LG, and Williams JA. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr 136: 1792-1799, 2006. PMID: 16772439.
- Sans MD, and Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am J Physiol Cell Physiol 287: C310-C319, 2004. PMID: 15044154.
- Sans MD, and Williams JA. The mTOR signaling pathway and regulation of pancreatic function. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2017. DOI: 10.3998/panc.2017.08.
- Sans MD, and Williams JA. Regulation of pancreatic protein synthesis and growth. In: The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery, edited by Beger HG, Warshaw AL, Hruban RH, Büchler MW, Lerch MM, Neoptolemos JP, Shimosegawa T, and Whitcomb DC. John Wiley & Sons Ltd., 2018, p. 95-105. DOI: 10.1002/9781119188421.ch9.
- Sans MD, and Williams JA. Translational control of protein synthesis in pancreatic acinar cells. International Journal of Gastrointestinal Cancer 31: 107-115, 2002. PMID: 12622421.
- Sans MD, Xie Q, and Williams JA. Regulation of translation elongation and phosphorylation of eEF2 in rat pancreatic acini. Biochem Biophys Res Commun 319: 144-151, 2004. PMID: 15158453.
- Santos CS, and Nascimento FEL. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: a biochemical review. Einstein (Sao Paulo) 17: eRB4898, 2019. PMID: 31508659.
- Scheele GA. Regulation of pancreatic gene expression in response to hormone and nutritional substrates. In: The Pancreas: Biology, Pathobiology, and disease, edited by Go VLW. New York: Raven Press, Ltd., 1993, p. 103-120.
- Schick J, Kern H, and Scheele G. Hormonal stimulation in the exocrine pancreas results in coordinate and anticoordinate regulation of protein synthesis. J Cell Biol 99: 1569-1574, 1984. PMID: 6208198.
- Schmid RM, and Meisler MH. Dietary regulation of pancreatic amylase in transgenic mice mediated by a 126-base pair DNA fragment. Am J Physiol Gastrointest Liver Physiol 262: G971-G976, 1992. PMID: 1377451.
- Schrader H, Menge BA, Belyaev O, Uhl W, Schmidt WE, and Meier JJ. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas 38: 416-421, 2009. PMID: 19169171.
- Schwarz DS, and Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73: 79-94, 2016. PMID: 26433683.
- Sehgal P, Szalai P, Olesen C, Praetorius HA, Nissen P, Christensen SB, Engedal N, and Moller JV. Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J Biol Chem 292: 19656-19673, 2017. PMID: 28972171.
- Snook JT. Effect of diet, adrenalectomy, diabetes, and actinomycin D on exocrine pancreas. Am J Physiol 215: 1329-1333, 1968. PMID: 5722992.
- Sofrankova A, and Dockray GJ. Cholecystokinin- and secretin-induced pancreatic secretion in normal and diabetic rats. Am J Physiol Gastrointest Liver Physiol 244: G370-G374, 1983. PMID: 6301289.
- Soling HD, and Unger KO. The role of insulin in the regulation of -amylase synthesis in the rat pancreas. Eur J Clin Invest 2: 199-212, 1972. PMID: 5053831.
- Srinivasan MP, Bhopale KK, Caracheo AA, Kaphalia L, Loganathan G, Balamurugan AN, Rastellini C, and Kaphalia BS. Differential cytotoxicity, ER/oxidative stress, dysregulated AMPKalpha signaling, and mitochondrial stress by ethanol and its metabolites in human pancreatic acinar cells. Alcohol Clin Exp Res 45: 961-978, 2021. PMID: 33690904.
- Srivastava SP, Davies MV, and Kaufman RJ. Calcium depletion from the endoplasmic reticulum activates the double-stranded RNA-dependent protein kinase (PKR) to inhibit protein synthesis. J Biol Chem 270: 16619-16624, 1995. PMID: 7622470.
- Steer ML. Pathogenesis of acute pancreatitis. Digestion 58 Suppl 1: 46-49, 1997. PMID: 9225091.
- Steer ML, and Saluja AK. Experimental acute pancreatitis: Studies of the early events that lead to cell injury. In: The Pancreas Biology, pathobiology and disease, edited by Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, and Scheele GA. New York, New York: Raven Press, Ltd., 1993, p. 489-500.
- Steinhilber W, Poensgen J, Rausch U, Kern HF, and Scheele GA. Translational control of anionic trypsinogen and amylase synthesis in rat pancreas in response to caerulein stimulation. Proc Natl Acad Sci U S A 85: 6597-6601, 1988. PMID: 2457915.
- Stockmann F, and Soling HD. Regulation of biosynthesis of trypsinogen and chymotrypsinogen by nutritional and hormonal factors in the rat. Eur J Clin Invest 11: 121-132, 1981. PMID: 6785096.
- Sung CK, and Williams JA. Insulin and ribosomal protein S6 kinase in rat pancreatic acini. Diabetes 38: 544-549, 1989. PMID: 2653925.
- Sutton R, Petersen OH, and Pandol SJ. Pancreatitis and calcium signalling: report of an international workshop. Pancreas 36: e1-14, 2008. PMID: 18437073.
- Sweiry JH, Emery PW, Munoz M, Doolabh K, and Mann GE. Influx and incorporation into protein of L-phenylalanine in the perfused rat pancreas: effects of amino acid deprivation and carbachol. Biochim Biophys Acta 1070: 135-142, 1991. PMID: 1751520.
- Szmola R, and Sahin-Toth M. Pancreatitis-associated chymotrypsinogen C (CTRC) mutant elicits endoplasmic reticulum stress in pancreatic acinar cells. Gut 59: 365-372, 2010. PMID: 19951900.
- Tabas I, and Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184-190, 2011. PMID: 21364565.
- Tadic V, Prell T, Lautenschlaeger J, and Grosskreutz J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci 8: 147, 2014. PMID: 24910594.
- Tahmasebi S, Khoutorsky A, Mathews MB, and Sonenberg N. Translation deregulation in human disease. Nat Rev Mol Cell Biol 19: 791-807, 2018. PMID: 30038383.
- Tashiro M, Samuelson LC, Liddle RA, and Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784-G790, 2004. PMID: 14684381.
- Trimble ER, Rausch U, and Kern HF. Changes in individual rates of pancreatic enzyme and isoenzyme biosynthesis in the obese Zucker rat. Biochem J 248: 771-777, 1987. PMID: 3325041.
- van Acker GJ, Perides G, and Steer ML. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J Gastroenterol 12: 1985-1990, 2006. PMID: 16610045.
- Van Acker GJ, Weiss E, Steer ML, and Perides G. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 292: G1738-G1746, 2007. PMID: 17332471.
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, and Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316-323, 2007. PMID: 17277771.
- Vassilakos A, Michalak M, Lehrman MA, and Williams DB. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry 37: 3480-3490, 1998. PMID: 9521669.
- Vesterhus M, Raeder H, Johansson S, Molven A, and Njolstad PR. Pancreatic exocrine dysfunction in maturity-onset diabetes of the young type 3. Diabetes Care 31: 306-310, 2008. PMID: 17989309.
- Wagenmakers AJ, Coakley JH, and Edwards RH. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int J Sports Med 11 Suppl 2: S101-113, 1990. PMID: 2193889.
- Walter P, and Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081-1086, 2011. PMID: 22116877.
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, and Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest 103: 27-37, 1999. PMID: 9884331.
- Wang WA, Groenendyk J, and Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta 1843: 2143-2149, 2014. PMID: 24440276.
- Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, and Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17: 5708-5717, 1998. PMID: 9755171.
- Waschulewski IH, Hall DV, Kern HF, and Edwardson JM. Effects of the immunosuppressants cyclosporin A and FK 506 on exocytosis in the rat exocrine pancreas in vitro. Br J Pharmacol 108: 892-900, 1993. PMID: 7683567.
- Webster PD, 3rd, Black O, Jr., Mainz DL, and Singh M. Pancreatic acinar cell metabolism and function. Gastroenterology 73: 1434-1449, 1977. PMID: 199525.
- Wei S, Zhao J, Wang S, Huang M, Wang Y, and Chen Y. Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon 4: e00830, 2018. PMID: 30294696.
- Wek RC, and Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal 9: 2357-2371, 2007. PMID: 17760508.
- Wek RC, Jiang HY, and Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7-11, 2006. PMID: 16246168.
- Wek S, Zhu S, and Wek R. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 15: 4497-4506, 1995. PMID: 7623840.
- Wicker C, and Puigserver A. Changes in mRNA levels of rat pancreatic lipase in the early days of consumption of a high-lipid diet. Eur J Biochem 180: 563-567, 1989. PMID: 2469576.
- Wicker C, and Puigserver A. Effects of inverse changes in dietary lipid and carbohydrate on the synthesis of some pancreatic secretory proteins. Eur J Biochem 162: 25-30, 1987. PMID: 3816784.
- Wicker C, Puigserver A, Rausch U, Scheele G, and Kern H. Multiple-level caerulein control of the gene expression of secretory proteins in the rat pancreas. Eur J Biochem 151: 461-466, 1985. PMID: 2411557.
- Williams CD, Oxon BM, and Lond H. Kwashiorkor: a nutritional disease of children associated with a maize diet. 1935. Bull World Health Organ 81: 912-913, 2003. PMID: 14997245.
- Williams JA. Cholecystokinin (CCK) regulation of pancreatic acinar cells: physiological actions and signal transduction mechanisms. Compr Physiol 9: 535-564, 2019. PMID: 30873601.
- Williams JA. Regulation of normal and adaptive pancreatic growth. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc.2020.05.
- Williams JA, and Goldfine ID. The insulin-acinar relationship. In: The Exocrine Pancreas: Biology, Pathobiology, and Diseases, edited by Go VLW. New York: Raven Press, 1986, p. 347-360.
- Williams JA, and Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 34: 980-986, 1985. PMID: 2412919.
- Williams JA, Sankaran H, Korc M, and Goldfine ID. Receptors for cholecystokinin and insulin in isolated pancreatic acini: hormonal control of secretion and metabolism. Fed Proc 40: 2497-2502, 1981. PMID: 6266879.
- Xiao F, Wang C, Yin H, Yu J, Chen S, Fang J, and Guo F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 7: 63679-63689, 2016. PMID: 27579768.
- Yagihashi S. Exocrine pancreas of streptozotocin-diabetes rats treated with insulin. Tohoku J Exp Med 120: 31-42, 1976. PMID: 134471.
- Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, and Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature 497: 217-223, 2013. PMID: 23636326.
- Yoshida H. ER stress and diseases. FEBS J 274: 630-658, 2007. PMID: 17288551.
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, and Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20: 6755-6767, 2000. PMID: 10958673.
- Yule DI. Ca2+ signaling in pancreatic acinar cells. In: Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc.2020.12.
- Yule DI. Cell Calcium. Special issue: Ca2+ signaling and secretory function. Preface. Cell Calcium 55: 281, 2014. PMID: 24813115.
- Zhang IX, Raghavan M, and Satin LS. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology 161: bqz028, 2020. PMID: 31796960.
- Zhang S, Zeng X, Ren M, Mao X, and Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8: 10, 2017. PMID: 28127425.
- Zhang Y, Nicholatos J, Dreier JR, Ricoult SJ, Widenmaier SB, Hotamisligil GS, Kwiatkowski DJ, and Manning BD. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 513: 440-443, 2014. PMID: 25043031.