Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2016.7
| Attachment | Size |
|---|---|
| 284.55 KB |
1. Importance of the pancreatic ductal HCO3- secretion
The exocrine pancreas secretes ~ 1.5 L of alkaline, isotonic fluid, which washes the digestive enzymes from the lumen of the pancreatic ducts and neutralizes the acidic gastric content entering the duodenum (4, 24). This alkaline pancreatic secretion plays an important role in the physiology and pathophysiology of the gland protecting the pancreatic tissue from damage. Findings from the last two decades supported this hypothesis and highlighted that the pancreatic acinar cells will suffer severe damage, if the pancreatic ductal secretion is impaired. Freedman et al. observed that in cftr knockout mice the pancreatic ductal secretion is impaired resulting in a more acidic (pH 6.6±0.04) pancreatic juice compared to wild type animals (pH 8.12±0.06) (13). In addition, the lack of cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel activity caused a defect in the apical membrane transport of the acinar cells. The findings of Reber et al. showed that in cat pancreas, the basal parenchymal pH was ~7.35, which decreased to ~7.25 after the induction of chronic pancreatitis (35). Moreover, ethanol administration decreased the extracellular pH of the pancreatic tissue to ~7.1 and reduced pancreatic blood flow to 40%. In a rat model, the development of acute pancreatitis (AP) was affected by the pH of the contrast solution during endoscopic retrograde cholangiopancreatography (28). Contrast solution at pH 6.0-6.9 injected into the main pancreatic ducts induced pancreatic oedema, increased serum amylase activity, neutrophil infiltration, and histological damage. The pancreatic injury correlated with the lower pH. On the other hand, pH 7.3 solution caused only mild pancreatic injury. Bhoomagoud et al. showed that the decrease of the extracellular pH from 7.6 to 6.8 augmented secretagogue-induced zymogen activation and acinar cell injury in vitro and enhanced cerulein-induced trypsinogen activation and pancreatic oedema in vivo (5). Our group further proved the importance of the pancreatic ductal secretion, since we demonstrated that the autoactivation of trypsinogen is a pH dependent process, with accelerated autoactivation on acidic pH meaning that HCO3- secretion protects the pancreas from untimely trypsinogen autoactivation (31). Evidence suggests that the decreased pancreatic ductal bicarbonate secretion can affect the severity of AP (see below).
2. Mechanism of the bicarbonate secretion in pancreatic ductal cells
The major site of the fluid and HCO3- secretion are the pancreatic ductal epithelial cells (PDEC) of the small intercalated and intralobular ducts (6). The maximal HCO3- concentration in the ductal lumen can vary among species; importantly human PDEC can produce 140 mM maximal intraluminal HCO3- concentration as can guinea pigs (4).
The complex process of pancreatic ductal HCO3- secretion can be divided into two steps: the accumulation of HCO3- across the basolateral membrane followed by the secretion via the apical membrane into the lumen. The basolateral accumulation of bicarbonate is mediated by the Na+/HCO3- cotransporter (NBCe1-B), which operates with 1 Na+ and 2 HCO3- stoichiometry (17). The passive diffusion of CO2 through the basolateral membrane may also contribute to the HCO3- accumulation, which is followed by the carbonic anydrase mediated conversion of CO2 to HCO3- (12). On the luminal membrane of the PDEC, the molecule central to HCO3- secretion are the electrogenic Cl-/HCO3- exchangers (SLC26A6 and possibly A3, which operates with a 1 Cl- : 2 HCO3- stoichiometry) (39). Another important protein is the CFTR Cl- channel, which plays an important role in the ductal HCO3- secretion in humans and animals, which produce a high intraluminal HCO3- concentration (45). This electrogenic apical Cl-/HCO3- exchange allows PDEC to transport HCO3- into the ductal lumen and establish 140 mM intraluminal HCO3- concentration during stimulated secretion (4, 24). The details and the molecular background of the pancreatic ductal HCO3- secretion have been reviewed recently elsewhere (1, 24, 27).
3. Effects of ethanol and ethanol metabolites on the pancreatic ductal bicarbonate secretion
One of the most common causes of AP is heavy alcohol abuse. The inhibitory effect of alcohol on pancreatic secretion was first suggested decades ago (16). In experimental studies, Yamamoto et al. found that 0.3-30 mM ethanol augmented, whereas 100 mM ethanol inhibited secretin-stimulated pancreatic ductal fluid secretion in the guinea pig (44). In the latter study, the authors focused on the effects of ethanol; however, numerous investigations have highlighted the harmful effects of different ethanol metabolites in different organs. In vivo ethanol metabolism is mediated by two independent pathways (23, 33). The oxidative pathway is predominant in the liver and generates acetaldehyde, whereas, the non-oxidative pathway combines ethanol and fatty acids (FA) and produces fatty acid ethyl esters (FAEE) in the pancreas, brain and heart, tissues typically damaged by excessive ethanol consumption (23). Compared with the liver, FAEE synthase activity in the pancreas is greater creating the possibility for the local accumulation of non-oxidative ethanol metabolites (15). FAEE can also be hydrolyzed leading to the intracellular accumulation of FA, which can strongly bind to mitochondrial membrane proteins and thus uncouple oxidative phosphorylation (22). Clinical studies (43) and experimental animal models suggest that ethanol administration in vivo does not induce pancreatitis by itself but sensitizes the pancreas to other triggers (32). Ethanol was shown to destabilize lysosomes and zymogen granules (42), to sensitize pancreatic mitochondria to activate mitochondrial permeability transition pore leading to mitochondrial failure (38), to modulate the immune response via sensitizing NF-κB activation in pancreatic acinar cells (37) and to cause oxidative ER stress, which activates an unfolded protein response and increases XBP1 levels and activity (25). Criddle et al. found that FAEE and FA, but not ethanol cause pancreatic acinar cell damage via sustained intracellular Ca2+ elevation, mitochondrial dysfunction, ATP depletion and intraacinar trypsinogen activation leading to cell necrosis (7, 8, 14, 34). Ethanol metabolites were also shown to perturb exocytosis processes in cultured rat pancreatic acini causing apical blockade and basolateral exocytosis (11). Moreover, Werner et al. showed that FAEE infusion induced significant increases in pancreatic edema, trypsinogen activation, and vacuolization of acinar cells (41). Recently the role of stellate cell activation has also been highlighted in the ethanol induced pancreatic injury (3); however, there is no direct evidence concerning the involvement of ductal epithelial cells in the pathogenesis of alcohol -induced pancreatitis.
Importantly, Sarles at al. described that the initial lesion in course of pancreatic damage during alcohol-induced chronic calcifying pancreatitis is the formation of mucoprotein plugs in the small pancreatic ducts (36). Besides this, the sweat chloride and sodium concentration of these patients were also significantly elevated compared to the control group (36). These changes are very similar to the alterations of the exocrine pancreas in cystic fibrosis, the most common genetic disorder in the Caucasian population, which was shown to cause exocrine pancreatic insufficiency (21) and increased risk of pancreatitis (29). Although the observations of Sarles are more than 50 years old, the connection of ethanol induced pancreatic damage and ductal secretory dysfunction has not been investigated in details yet.
Recently, we demonstrated using several overlapping in vivo and in vitro experimental methods that ethanol and FA dose-dependently reduced CFTR expression and activity in PDEC, and inhibited secretion of fluid and HCO3- in the pancreas (18, 26). We observed that the sweat Cl- concentration (Cl-sw) was significantly elevated after heavy alcohol intake in human subjects; however, the Cl-sw normalized when the patients were sober (26). In human tissue samples from patients suffering from alcohol-induced acute or chronic pancreatitis, we detected a significant decrease of CFTR expression at the apical membrane of the pancreatic ducts. Interestingly, in experimental models we found that low concentration (10 mM) of ethanol stimulated both the apical Cl-/HCO3- exchange and the CFTR channel activities. However, at high concentration (100 mM) a strong inhibitory effects were detected on HCO3- secretion, CFTR activity and pancreatic fluid secretion in vivo and in vitro. This dual effect of ethanol is very similar to the dose-dependent effects of non-conjugated bile acids on the pancreatic ductal functions (40). Similarly to 100 mM ethanol, FA impaired pancreatic fluid and HCO3- secretion. The oxidative ethanol metabolite acetaldehyde and FAEE had no such effects. The inhibition of CFTR by ethanol and FA was associated with a sustained increase in concentrations of intracellular Ca2+ and decreased 3',5'-cyclic adenosine monophosphate (cAMP) levels, mitochondrial membrane depolarization, and a consequent drop of intracellular ATP levels. Intracellular ATP supplementation via a patch pipette almost completely prevented inhibition of CFTR activity by ethanol and FA (18). We also showed that the decrease in CFTR expression and plasma membrane density in response to ethanol, palmitoleic acid, or palmitoleic acid ethyl ester administration was caused by the combination of accelerated plasma membrane turnover at the apical membrane and by damaged protein folding in the endoplasmic reticulum (26).
4. Alcohol-induced CFTR dysfunction in the pathogenesis of pancreatic damage
As demonstrated above high concentrations of ethanol and ethanol metabolites have a strong inhibitory effect on the pancreatic HCO3- and fluid secretion via the reduced function and expression of CFTR (Figure 1). In addition to these experimental observations, other data suggest that CFTR function can affect the pathogenesis and severity of AP.
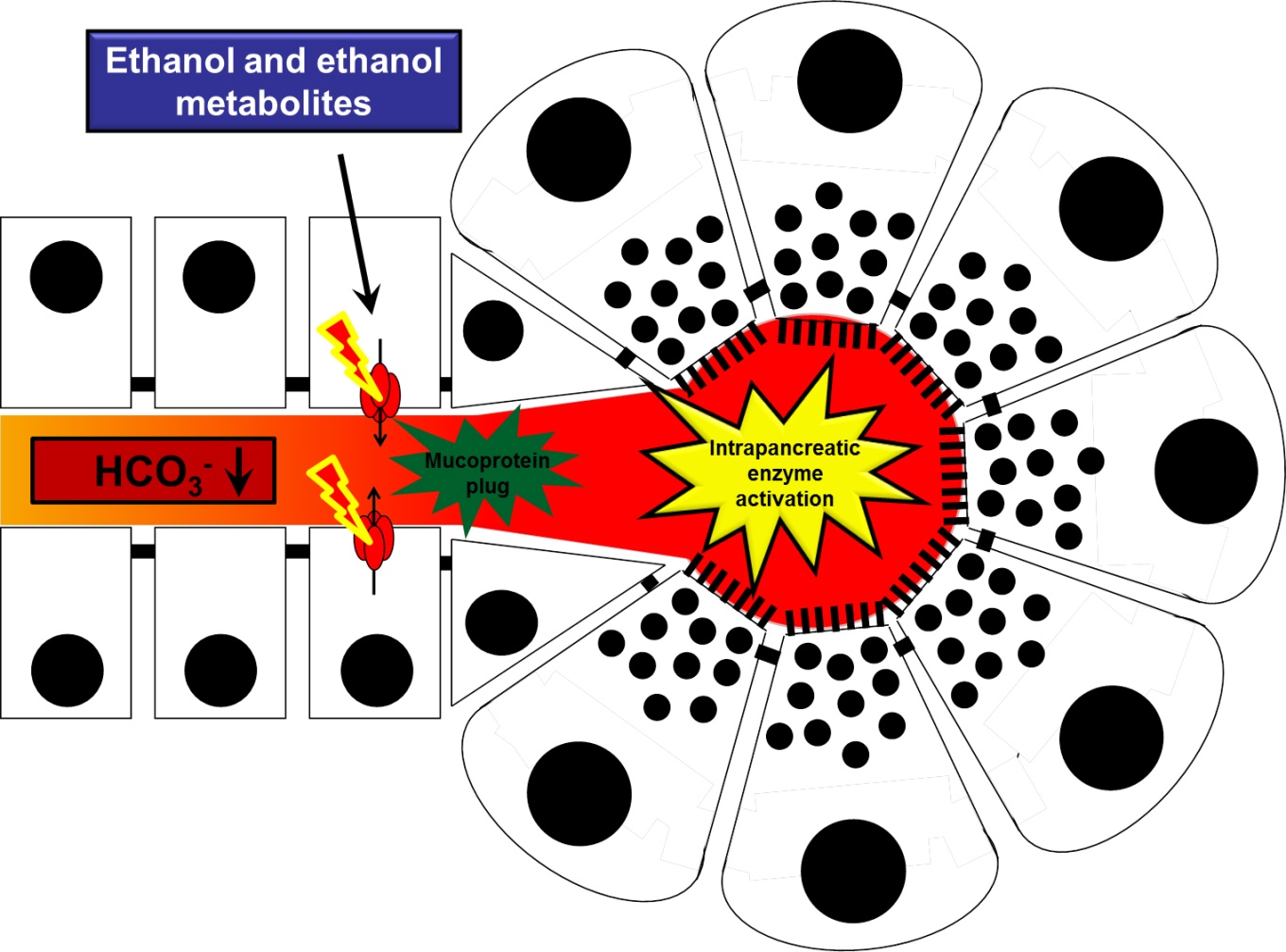
Figure 1. The effects of ethanol and ethanol metabolites on pancreatic ductal function. Under physiological conditions, CFTR Cl- channel (red) is expressed on the luminal membrane of small inter/intralobular pancreatic ducts and contributes significantly to the pancreatic HCO3- secretion, which maintains the alkaline intraluminal pH. During acute or chronic alcohol-induced pancreatitis, the function and expression of CFTR is markedly reduced by ethanol and ethanol metabolites, which leads to impaired HCO3- and fluid secretion and consequently decreased intraluminal pH. Under these conditions, the wash out of the luminal content is insufficient promoting the formation of intraluminal protein plugs. The intraductal obstruction will lead to intrapancreatic enzyme activation in acute pancreatitis and to pancreatic atrophy and exocrine pancreatic insufficiency in chronic pancreatitis.
DiMagno et al. showed that deletion of CFTR results in continuous overexpression of proinflammatory cytokine genes, moreover these mice develop more severe AP upon cerulein hyperstimulation compared to wild type animals (9). They observed elevated pancreatic edema, neutrophil infiltration and mRNA expression of multiple inflammatory mediators; however, acinar cell injury was not different. On the other hand acinar cell apoptosis in cftr knockout mice was decreased in cftr knockout mice, which also had mild exocrine pancreatic insufficiency (as pointed out by impaired in vivo pancreatic secretion in response to cholecystokinin and reduced pancreatic digestive enzyme protein and mRNA levels). These results were reproduced in ΔF508 cftr mutant mice (10). These observations are important, although the authors focused on the alterations of acinar cells, whereas CFTR is expressed on the apical membrane of pancreatic ductal cells. The lack of pancreatic CFTR expression impairs the ductal fluid and bicarbonate secretion and any alterations of the acinar cells might be presumably indirect. Recently, our group demonstrated that cftr knockout mice displayed more severe AP induced by i.p. injection of ethanol and palmitic acid (26). All laboratory and histological parameters were significantly elevated in cftr knockout mice compared to wild type controls, including the extension of necrosis. These data have potential clinical relevance as well, since we detected markedly decreased CFTR protein and mRNA expression in small pancreatic ducts using pancreatic tissue samples from patients diagnosed with alcohol-induced AP (26). Another study by Pallagi et al. confirmed the potential role of CFTR and pancreatic ductal secretion in the pathogenesis of AP (30). In the latter study, Na+/H+ exchanger regulatory factor-1 (NHERF-1, a cytosolic scaffolding protein involved in the apical targeting and retention of membrane proteins) knockout mice were used, which had lower CFTR expression in the apical membrane of pancreatic ducts and lower pancreatic bicarbonate and fluid secretion. Cerulein hyperstimulation and sodium taurocholate infusion into the pancreas induced more severe pancreatitis further confirming the importance of CFTR-mediated pancreatic secretion.
On the other hand, alcohol-induced CFTR dysfunction and therefore impaired HCO3- secretion seems to be involved not just in the pathogenesis of AP, but also in chronic pancreatitis (CP). In CP, the destruction of the pancreas can be observed due to chronic inflammation, exocrine pancreatic insufficiency, decreased pancreatic fluid and bicarbonate secretion, fibrosis and calcification of the tissue. As an underlying mechanism for the decreased secretion, CFTR dysfunction due to mislocalised protein expression in pancreatic ductal cells has been observed in different forms of CP. Using human pancreatic tissue samples, Ko et al. described that CFTR is mislocalised in alcoholic, obstructive and idiopathic chronic pancreatitis as well similarly to our results (20). The decreased expression of CFTR, observed in different forms of chronic pancreatitis, could explain the impaired function of the PDEC (20). The impaired fluid and HCO3- secretion lead to decreased intraluminal pH, decreased wash out of the digestive enzymes and more viscous, protein-rich ductal fluid (Figure 1) (19). These changes promote the formation of intraluminal protein gel, or plugs that are one of the earliest histological features of chronic pancreatitis (36). The intraductal obstruction can lead to pancreatic atrophy, ductal mucinous hyperplasia (2), Goblet-cell metaplasia and the protein plugs might also underlie pancreatic stone formation (19).
Acknowledgements
This work was supported by the Hungarian Scientific Research Fund (K116634 to PH, K109756 to VV and PD115974 to MJ) and the Momentum Grant of the Hungarian Academy of Sciences (LP2014-10/2014 to P.H).
5. References
- Ahuja M, Jha A, Maleth J, Park S and Muallem S. cAMP and Ca(2)(+) signaling in secretory epithelia: crosstalk and synergism. Cell Calcium 55(6): 385-393, 2014. PMID: 24613710.
- Allen-Mersh TG. What is the significance of pancreatic ductal mucinous hyperplasia? Gut 26(8): 825-833, 1985. PMID: 4018649.
- Apte MV, Pirola RC and Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol 25(12): 1816-1826, 2010. PMID: 21091991.
- Argent BE GM, Steward MC, Case MR. Cell Physiology of Pancreatic Ducts. Physiology of the Gastronintestinal Tract. Johnson LL. Oxford, Academic Press. 5: 1399-1424, 2012.
- Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, et al. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology 137(3): 1083-1092, 2009. PMID: 19454288.
- Bolender RP. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol 61(2): 269-287, 1974. PMID: 4363955.
- Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology 130(3): 781-793, 2006. PMID: 16530519.
- Criddle DN, Raraty MG, Neoptolemos JP, Tepikin AV, Petersen OH and Sutton R. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci U S A 101(29): 10738-10743, 2004. PMID: 15247419.
- Dimagno MJ, Lee SH, Hao Y, Zhou SY, McKenna BJ and Owyang C. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (-/-) mice. Gastroenterology 129(2): 665-681, 2005. PMID: 16083720.
- DiMagno MJ, Lee SH, Owyang C and Zhou SY. Inhibition of acinar apoptosis occurs during acute pancreatitis in the human homologue DeltaF508 cystic fibrosis mouse. Am J Physiol Gastrointest Liver Physiol 299(2): G400-412, 2010. PMID: 20522641.
- Dolai S, Liang T, Lam PP, Fernandez NA, Chidambaram S and Gaisano HY. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology 143(3): 832-843 e831-837, 2012. PMID: 22710192.
- Dyck WP, Hightower NC and Janowitz HD. Effect of acetazolamide on human pancreatic secretion. Gastroenterology 62(4): 547-552, 1972. PMID: 5020866.
- Freedman SD, Kern HF and Scheele GA. Pancreatic acinar cell dysfunction in CFTR(-/-) mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121(4): 950-957, 2001. PMID: 11606508.
- Gerasimenko JV, Lur G, Ferdek P, Sherwood MW, Ebisui E, Tepikin AV, et al. Calmodulin protects against alcohol-induced pancreatic trypsinogen activation elicited via Ca2+ release through IP3 receptors. Proc Natl Acad Sci U S A 108(14): 5873-5878, 2011. PMID: 21436055.
- Gukovskaya AS, Mouria M, Gukovsky I, Reyes CN, Kasho VN, Faller LD, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology 122(1): 106-118, 2002. PMID: 11781286.
- Hajnal F, Flores MC and Valenzuela JE. Pancreatic secretion in chronic alcoholics. Effects of acute alcohol or wine on response to a meal. Dig Dis Sci 38(1): 12-17, 1993. PMID: 8420743.
- Ishiguro H, Steward MC, Lindsay AR and Case RM. Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495 ( Pt 1): 169-178, 1996. PMID: 8866360.
- Judak L, Hegyi P, Rakonczay Z, Jr., Maleth J, Gray MA and Venglovecz V. Ethanol and its non-oxidative metabolites profoundly inhibit CFTR function in pancreatic epithelial cells which is prevented by ATP supplementation. Pflugers Arch 466(3): 549-562, 2014. PMID: 23948742.
- Ko SB, Azuma S, Yoshikawa T, Yamamoto A, Kyokane K, Ko MS, et al. Molecular mechanisms of pancreatic stone formation in chronic pancreatitis. Front Physiol 3: 415, 2012. PMID: 23133422.
- Ko SB, Mizuno N, Yatabe Y, Yoshikawa T, Ishiguro H, Yamamoto A, et al. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology 138(5): 1988-1996, 2010. PMID: 20080093.
- Kristidis P, Bozon D, Corey M, Markiewicz D, Rommens J, Tsui LC, et al. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet 50(6): 1178-1184, 1992. PMID: 1376016.
- Lange LG and Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest 72(2): 724-731, 1983. PMID: 6308061.
- Laposata EA and Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 231(4737): 497-499, 1986. PMID: 3941913.
- Lee MG, Ohana E, Park HW, Yang D and Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 92(1): 39-74, 2012. PMID: 22298651.
- Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 140(3): 987-997, 2011. PMID: 21111739.
- Maleth J, Balazs A, Pallagi P, Balla Z, Kui B, Katona M, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 148(2): 427-439 e416, 2015. PMID: 25447846.
- Maleth J and Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium 55(6): 337-345, 2014. PMID: 24602604.
- Noble MD, Romac J, Vigna SR and Liddle RA. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut 57(11): 1566-1571, 2008. PMID: 18625695.
- Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 140(1): 153-161, 2011. PMID: 20923678.
- Pallagi P, Balla Z, Singh AK, Dosa S, Ivanyi B, Kukor Z, et al. The role of pancreatic ductal secretion in protection against acute pancreatitis in mice*. Crit Care Med 42(3): e177-188, 2014. PMID: 24368347.
- Pallagi P, Venglovecz V, Rakonczay Z, Jr., Borka K, Korompay A, Ozsvari B, et al. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl(-) channels and luminal anion exchangers. Gastroenterology 141(6): 2228-2239 e2226, 2011. PMID: 21893120.
- Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 117(3): 706-716, 1999. PMID: 10464148.
- Patton S and McCarthy RD. Conversion of alcohol to ethyl esters of fatty acids by the lactating goat. Nature 209(5023): 616-617, 1966. PMID: 5950784.
- Petersen OH, Tepikin AV, Gerasimenko JV, Gerasimenko OV, Sutton R and Criddle DN. Fatty acids, alcohol and fatty acid ethyl esters: toxic Ca2+ signal generation and pancreatitis. Cell Calcium 45(6): 634-642, 2009. PMID: 19327825.
- Reber HA, Karanjia ND, Alvarez C, Widdison AL, Leung FW, Ashley SW, et al. Pancreatic blood flow in cats with chronic pancreatitis. Gastroenterology 103(2): 652-659, 1992. PMID: 1634080.
- Sarles H, Sarles JC, Camatte R, Muratore R, Gaini M, Guien C, et al. Observations on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut 6(6): 545-559, 1965. PMID: 5857891.
- Satoh A, Gukovskaya AS, Reeve JR, Jr., Shimosegawa T and Pandol SJ. Ethanol sensitizes NF-kappaB activation in pancreatic acinar cells through effects on protein kinase C-epsilon. Am J Physiol Gastrointest Liver Physiol 291(3): G432-438, 2006. PMID: 16574982.
- Shalbueva N, Mareninova OA, Gerloff A, Yuan J, Waldron RT, Pandol SJ, et al. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 144(2): 437-446 e436, 2013. PMID: 23103769.
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, et al. Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6. J Gen Physiol 127(5): 511-524, 2006. PMID: 16606687.
- Venglovecz V, Rakonczay Z, Jr., Ozsvari B, Takacs T, Lonovics J, Varro A, et al. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut 57(8): 1102-1112, 2008. PMID: 18303091.
- Werner J, Laposata M, Fernandez-del Castillo C, Saghir M, Iozzo RV, Lewandrowski KB, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology 113(1): 286-294, 1997. PMID: 9207289.
- Wilson JS, Apte MV, Thomas MC, Haber PS and Pirola RC. Effects of ethanol, acetaldehyde and cholesteryl esters on pancreatic lysosomes. Gut 33(8): 1099-1104, 1992. PMID: 1398235.
- Yadav D and Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144(6): 1252-1261, 2013. PMID: 23622135.
- Yamamoto A, Ishiguro H, Ko SB, Suzuki A, Wang Y, Hamada H, et al. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol 551(Pt 3): 917-926, 2003. PMID: 12847207.
- Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol 273(2 Pt 1): C442-455, 1997. PMID: 9277342.


