Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2013.23
| Attachment | Size |
|---|---|
| 362.65 KB |
Gene symbols: Gcg
1. General Information
In 1907, Michael Lane described his anatomical observations of the pancreatic islets of Langerhans as cells with distinctive anatomical properties versus the earlier theory as those of “exhausted acini”(58). In his characterization of stained hamster pancreatic sections, Lane described the existence of two distinct types of cells within the islet, which he referred to as the large α and smaller β cells (58). Four decades later, Sutherland and de Duve established that the α-cells of the pancreatic islet are the primary source of glucagon (95,96). Initially labeled as a contaminant, the physiological role of glucagon was first described in 1921, when Banting and Best conducted their classical experiments of insulin’s actions. Banting and Best tested their first pancreatic extracts in depancreatized dogs and observed an initial transient rise in blood glucose followed by the insulin-induced hypoglycemia (36). Several years later, Murlin and his colleagues credited this transient rise in blood glucose to a hormone they named “Glucagon” or “Hyperglycemic-Glycogenolytic Factor” (28,36,71). Insights into the regulation of glucagon release came from elegant cross-circulation experiments performed by Foa and his colleagues in the 1950s, showing that hypoglycemia triggered by the injection of insulin in a donor dog induces the release of glucagon, which secreted through the donor blood via a pancreatic-femoral anastomosis causes a hyperglycemic response in a recipient dog (28). Bensley and Woerner added to these observations with their suggestion that glucagon induces liver glycogenolysis and thereby promotes a rise in blood glucose levels (4).
Glucagon is a 29-amino acid peptide derived from the tissue specific-processing of proglucagon in pancreatic α-cells through cleavage by prohormone convertase 2 (PCSK2) (Figure 1) (30). In contrast, processing of proglucagon to glucagon-like peptides (GLP-1, GLP-2), oxyntomodulin and glicentin occurs in intestinal enteroendocrine cells ( Figure 1) (62). Glucagon plays a major role in antagonizing the effects of insulin and maintaining glucose homeostasis by promoting hepatic gluconeogenesis and glycogenolysis and inhibiting glycogen synthesis. Therefore, the secretion of glucagon is normally induced in states of decreasing blood glucose, such as fasting and increased energy expenditure, to sufficiently induce a rapid, yet transient, rise in blood glucose. However, aberrant secretion of glucagon is a characteristic of Types I and II Diabetes. Increased secretion of glucagon is observed in patients with Type II Diabetes, leading to an increase in hepatic glucose output and exacerbating the hyperglycemic state (19,63,79,84,106,107). In contrast, failure to secrete adequate glucagon in response in hypoglycemia is a limiting factor for glucose control, contributing to the morbidity and mortality of patients with Type I Diabetes (15,33).
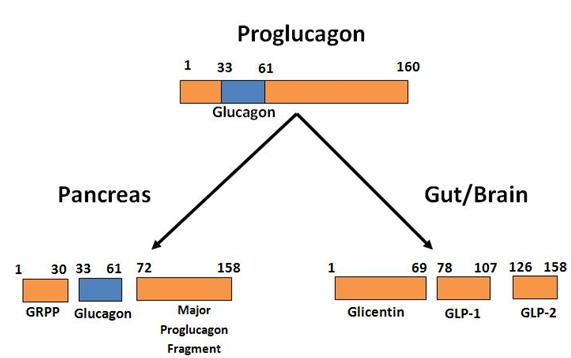
Figure 1. Diagram of differential processing of the proglucagon gene product in α-cells of the pancreas, gut (L-cells) and brain. Only biologically active products are shown. For further details of cleavage sites and processing see reference (62).
2. Glucagon and the Endocrine Pancreas
The pancreatic islet comprises five major types of polypeptide-secreting cells: insulin-secreting β-cells (65-80% of total islet cells), the glucagon secreting α-cells (15-20%), somatostatin-secreting δ-cells (3-10%) and pancreatic polypeptide-secreting cells (3-5%), along with ghrelin-positive cells which are mostly observed in early development (103). The five major islet cell types are aligned on blood vessels at no particular order or structured organization within the human islet (8) (Figure 2A). In contrast, the rodent islet shows a more-defined architecture placing the β-cells in the core and the α, δ and PP-cells lying at the mantle of the islet (Figure 2B). This unique structure in the rodent islet suggests an organized system allowing paracrine interactions between the peptides released. This is supported by studies showing that arterial blood is directed from the core of the rodent islet (insulin-secreting β-cells) to the periphery (6). Therefore, during a rise in blood glucose, the pancreatic α-cells are exposed to high levels of secreted insulin leading to the inhibition of glucagon secretion and glucagon gene transcription.
3. Regulation of Glucagon Release
The secretion of glucagon by the α-cells is regulated by the effects of paracrine/endocrine factors as wells as neuronal inputs. Glucagon release is inhibited after carbohydrate-rich meal and the consequent rise in blood glucose and insulin secretion. However, a meal rich in amino acids induces glucagon release. Parasympathetic (vagal) and sympathetic (Epinephrine, Norepinephrine, Galanin, Neuropeptide Y) nerve stimulations induce the secretion of glucagon from the pancreatic α-cells.
Glucose
Glucagon is secreted by the pancreatic α-cells in states of decreasing blood glucose; however, whether changes in glucose concentrations alone can regulate glucagon secretion still remains unclear. High glucose concentrations inhibit glucagon release in the intact islet; however, high glucose induces glucagon release in dispersed, isolated α-cells. These data support the notion that the paracrine interactions of the islet cells, in particular the secreted insulin/GABA and somatostatin, act as primary regulators by suppressing glucagon secretion in hyperglycemia (76). However, the chronic exposure of α-cells to high glucose levels has been shown to induce α-cell dysfunction and insulin-resistance, closely mimicking the diabetic state (21, 92).
Rat α-cells express glucokinase and glucose transporter GLUT1, an isoform with a lower capacity compared to GLUT2, which is the predominant form in insulin-secreting β-cells (42). Despite differences in metabolism of glucose by the two cells types, studies have shown that they share similar inherent mechanisms of activation (76). Alpha cells have high ATP concentrations under low glucose, which rise further after stimulation with high glucose. The glucose-stimulated inhibition of glucagon secretion was associated with an inhibition of AMPK activity and activating AMP-activated protein kinase (AMPK) in turn inhibited secretion (59). Therefore, α-cells do have intrinsic mechanisms, which respond to glucose stimulation, but synergize with extrinsic paracrine signals to regulate secretion under high glucose stimulation (31).
Contrary to the regulation of glucagon release in high glucose conditions, it has been shown that extrinsic paracrine signals (insulin/GABA, somatostatin) do not play a role in regulating glucagon secretion in low glucose concentration (1-6 mM) (99). This is supported by in vivo findings showing that inhibiting insulin signaling in the pancreatic α-cells of mice through a loss of α-cell insulin receptors leads to an increase in glucagon secretion in both hyperinsulimic-hypoglycemic and STZ-induced hypoinsulimic-hyperglycemic state. Therefore, these data shows that insulin is necessary for inhibiting glucagon secretion in hyperglycemia; however, insulin does not play a role in regulating glucagon secretion in low-glucose conditions (52).
Insulin and GABA
Pancreatic α-cells are exposed to high levels of insulin secreted from the β-cells in the islet. Insulin is a potent inhibitor of glucagon secretion and glucagon gene transcription (2,67,78,101,107). Data have shown that the diminished insulin release during hyperglycemia associated with diabetes paradoxically stimulates the release of glucagon (19,79,106). Studies utilizing in vitro approaches have shown that insulin receptors are very abundant on pancreatic α-cells and activate the phosphatidyl inositol 3-kinase (PI3K)-Akt pathway leading to inhibition of glucagon gene transcription and secretion (82,84,105). Insulin has been shown to induce the Akt-dependent GABAA receptor translocation to the plasma membrane (which can be activated by GABA (co-released with insulin) and PI3K-dependent opening of KATP channels, culminating hyperpolarizing the plasma membrane and inhibiting glucagon secretion (29,105). Although, the precise mechanisms responsible for changes in the α-cell function in diabetes remain unclear, a recent study by Kawamori and colleagues showed that inhibiting insulin signaling in the pancreatic α-cells of mice through a loss of α-cell insulin receptors leads to altered glucose metabolism, including mild glucose intolerance, hyperglycemia and hyperglucagonemia (52).
GABA (γ-Aminobutyric acid) is produced from the excitatory amino acid glutamate and co-released with insulin from the pancreatic β-cells by high glucose and glutamate stimulation. GABA can diffuse within the islet interstitium to activate GABAA receptors present on the cell-surface of α-cells (102). This nonpeptidal neurotransmitter has been shown to act as a suppressor of amino acid-stimulated glucagon release in the mouse and isolated α-cells via GABAA receptors (35). Data have suggested that glucose-stimulated insulin release and the subsequent activation of the Insulin Receptor-PI3K-Akt pathway induces the activation and translocation of GABAA receptors to the plasma membrane (105). The GABA co-released with insulin from the β-cells can activate the newly translocated cell-surface GABAA receptors and increase Cl- inhibitory currents, subsequently hyperpolarizing the plasma membrane (102). The hyperpolarization of the membrane closes the voltage-dependent Ca2+ channels, which lowers the free cytoplasmic Ca2+ levels and reduces glucagon exocytosis. (80,102).
Glucagon and Glutamate
Perfusion experiments in the human and rat pancreas have shown that glucagon suppresses insulin and somatostatin release (7,94). Glucagon receptor knock-out mice exhibit α-cell hyperplasia and hyperglucagonemia, which has been suggested to be due to a lack of autocrine signals of glucagon on the α-cell (36). Contrary to this theory, a recent study has shown that implanted wild-type islets in mice with liver-specific deletion of the glucagon receptor also develop α-cell hyperplasia (64). These data suggest that a circulating factor generated after the disruption of glucagon signaling in the liver can increase α-cell proliferation independent of direct pancreatic input.
Glutamate is a major excitatory neurotransmitter in the central nervous system, which has also been implicated in the regulation of glucagon release. An elegant study published by Cabrera and colleagues described the positive autocrine signal of glutamate in the human, monkey and mouse islets (9). The authors proposed a mechanistic model where glutamate co-released with glucagon potentiates glucagon secretion through acting on the inotropic glutamate receptors on the α-cell membrane and creating a positive autocrine loop (9).
Somatostatin
Somatostatin, secreted by the islet δ-cells, has been long accepted as a glucagon-suppressing peptide. Exogenous somatostatin inhibits glucagon release in isolated α-cells, as wells as in healthy and diabetic patients (11,34). In addition, islets isolated from somatostatin-deficient mice have reduced glucose-suppression of glucagon release (41).
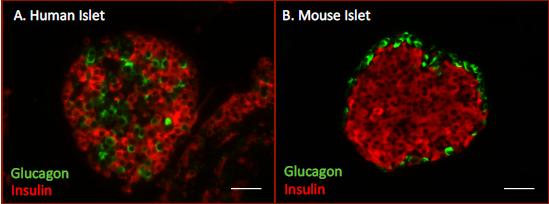
Figure 2. Immunofluorescent labeling of human and mouse islets. A. Human islet. Glucagon-positive α-cells (green) are randomly dispersed among insulin-positive β-cells (red) within the human islet. B. Mouse islet. Glucagon-positive α-cells (green) are concentrated on the mantle and insulin-positive β-cells (red) make-up the core of the mouse islet.
4. Physiological Actions of Glucagon at Target Tissues
Glucagon exerts its physiological action on target tissues via the G-protein coupled glucagon receptor, which is found on multiple tissues including the liver, fat, intestine, kidney and brain (50,68).
In the liver, glucagon counteracts the anabolic properties of insulin by promoting gluconeogenesis and glycogenolysis which consequently increases glucose output. The hepatocyte is exposed to high levels of glucagon released by the pancreas via the portal vein. The mechanistic actions of glucagon on the hepatocyte are mediated by glucagon’s binding and activation of the glucagon receptor on the cell membrane, which upon conformation change activates G proteins. The subsequent activation of adenylate cyclase leads to the increase in intracellular cyclic adenosine monophosphate (cAMP) levels and the activation of protein kinase A (PKA) (50). The second messenger cAMP can activate cyclic nucleotide-gated ion channels, exchange proteins activated by cAMP (EPAC) and protein kinase A (PKA). Glucagon’s activation of the liver’s glucagon receptor leading to hepatic glucose output has been shown to act through PKA’s activation and phosphorylation of downstream gluconeogenic enzymes, although it’s unclear to what extent the other cAMP downstream pathways may also play a role in this process. The process of glycogenolysis involves the activation of glycogen phosphorylase kinase and glycogen phosphorylase through the activated PKA, and glycogen phosphorylase brings about glycogen breakdown. In addition, the activation of the cAMP-PKA pathway in the hepatocyte leads to the phosphorylation and activation of CREB and subsequent activation of key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), stimulating hepatic glucose output. The gluconeogenic process is also promoted by the activity of additional transcription factors. The activated CREB binds to the promoter region of the transcriptional coactivator PGC-1 gene, increasing its transcription. PGC-1 and the nuclear transcription factor hepatocyte nuclear factor-4 (HNF-4) further promote gluconeogenesis by increasing the transcription of PEPCK gene and therefore PEPCK activity 50).
In the adipocyte, glucagon activates the cAMP-PKA pathway, leading to the phosphorylation and activation of hormone-sensitive lipase and the subsequent breakdown of triglycerides (lipolysis) and release of diacylglycerol and free fatty acids into the circulation. The liver can further utilize glycerol and free fatty acids for gluconeogenesis or re-esterification of free fatty acids to form ketone bodies.
Glucagon’s physiological effects on the heart and kidney have also been described, although glucagon’s actions in these tissues are less understood mechanistically compared to those in the liver and fat. In the heart, glucagon has been described as a vasodilator, which lowers blood pressure by decreasing the vasculature resistance in the liver and spleen. Glucagon has diuretic effects on the kidney, increasing glomerular filtration and electrolyte excretion (26).
Glucagon receptors are expressed in the brain and data has suggested that circulating glucagon can pass the blood-brain barrier to modulate its effects in the central nervous system. Glucagon infused in the central nervous system has anorexigenic effects in rats, chicks and sheep. In addition, intravenous infusion of glucagon has been shown to suppress appetite in humans; however, the direct link between glucagon and central food intake regulation in humans is unclear. Although, the mechanisms of glucagon’s activity on the brain remain largely unclear, the involvement of the hypothalamic corticotropin-releasing factor (CRF) and the activation of the hypothalamic-pituitary-adrenal (HPA) axis are implicated to be involved in modulating glucagon’s suppression of food intake (27).
Glucagon relaxes the GI tract from esophagus to colon and as a result is often used to quiet the bowel before endoscopic retrograde cholangiopancreatography (ERCP) or bowel imaging studies (69). In the esophagus it is used to relax the muscle before removal of foreign objects. Glucagon also will relax the sphincter of Oddi (94). These effects are almost certainly pharmacological but are short lived and without other deleterious effects.
5. Glucagon and the Exocrine Pancreas
Crystaline glucagon was first prepared in 1953 and early reports injecting mg amounts of glucagon into rodents included a description of degranulation of acinar cells along with pancreatic atrophy (10,49,57,81). This finding was interpreted by Jarett as due to inhibition of protein synthesis due to lowered plasma amino acid levels in vivo as glucagon did not inhibit protein synthesis measured by incorporation of 3H-leucine in vitro (48). However, these results are difficult to interpret because the animals given megadoses of glucagon lost weight and were reported to appear ill. In a subsequent and more sophisticated study, Adler (1) infused glucagon IV into rats at 10-80 μg/kg/hr and found that the pancreas lost 80% of three digestive enzymes after 24 hours and EM showed viable cells but with few granules. However, the 50 μg/kg/hr equated to 1.2 mg glucagon in 24 hours and clearly represented a non-physiological treatment. In vitro studies with isolated lobules showed that in-vivo pretreatment reduced subsequent protein synthesis and intracellular transport after 30 minutes of infusion in vivo but not at 24 hours. In a more recent study, Kash et. al (57) reported that 30 μg/kg of glucagon every 8 hours reduced the trophic effect of caerulein over a 5 day period, but on its own did not affect pancreatic mass, protein or DNA.
With the knowledge of a possible relationship between the endocrine and exocrine pancreas (43), the effects of exogenous glucagon on pancreatic secretion was studied in a variety of species both with and without anesthesia. Initially most studies were carried out in unanesthetized dogs with pancreatic fistulas, the predominant animal model for GI physiology at the time, and glucagon (most often prepared by Eli Lilly) was shown to inhibit the volume, bicarbonate and protein or enzyme content of pancreatic secretion stimulated by food, acid, secretin or CCK (24,44,45,47,55,56,73,74,85). In most cases a large amount of glucagon was used (20-30 μg/kg/hr) and resulting plasma levels of glucagon were not measured or related to physiological levels. Similar inhibition of pancreatic secretion has also been seen in studies carried out in rats (1,5,83), cats (54) and humans (12,18,25,40,53). The mechanism of the inhibition remains unclear, but has been assumed by most authors to be at the level of the pancreas because the effect of exogenous secretagogues was inhibited. Possible loci include inhibition of pancreatic blood flow, the resulting hyperglycemia, lowering of plasma calcium as well as inhibition of the secretory mechanism. Glucagon could also be having an effect on the nervous system either centrally or within the pancreas.
Some of these possible inhibitory loci could be better controlled using a perfused pancreas model. Glucagon has been reported to inhibit secretion in the perfused pancreas of the cat (104) and rat (98). In the latter study, infusion of amino acids was shown to increase glucagon and inhibit pancreatic secretion and this effect could be blocked by infusing an antibody to glucagon (98). Other studies, however, gave different results. In a study in the perfused dog pancreas glucagon had no effect (72), but in a study in perfused rat pancreas, glucagon increased the basal flow and protein output. However, when glucagon was combined with secretin, it decreased the volume and protein output (91). Other in vitro studies have been carried out using pancreatic segments or lobules. In studies of rat pancreas lobules, glucagon increased amylase secretion and potentiated effects of acetylcholine, CCK or electric field stimulation to activate nerves (86,87). By contrast, glucagon was reported to increase amylase release from mouse segments (66) or have no effect on in vitro release of amylase by mouse (16) or rat pancreatic fragments (1). While all of the positive in vitro studies imply a direct effect on the pancreas, there is no clear overall pattern.
With the development of isolated pancreatic acini and isolated acinar cells, the effects of glucagon were studied and compared to the structurally related peptides, secretin and VIP. Natural purified glucagon was shown to stimulate amylase release and increase cyclic AMP at high concentrations (1 to 100 µM) in isolated acini from guinea pig, rat and mouse acini (77,89,90). The effect was similar to that of secretin, but observed at much higher concentrations. The material did not interact with VIP receptors on acini and did not elute with purified synthetic glucagon, so its nature is unknown. Most importantly, synthetic glucagon had no effect on amylase secretion (3,77). Glucagon receptor mRNA has been identified in pancreas and in isolated islets by several techniques (22,39,70). However, none of these studies specifically evaluated exocrine pancreas or showed receptor mRNA in acini or ducts. In summary, the studies of glucagon on isolated acini and the current state of receptor knowledge do not support a direct effect of glucagon on acinar cells. Moreover, some of the stimulatory effects of glucagon on isolated perfused pancreas or pancreatic fragments could have been due to a contaminant in natural glucagon.
Because of the effect of glucagon to inhibit secretion in animals and humans, it was studied as a possible therapeutic agent in pancreatitis. In animal models, glucagon protected against hemorrhagic pancreatitis in mice, but only when given before the inducing choline-deficient ethionine-supplemented (CDE) diet (65). In pigs with retrograde injection of bile salts, glucagon infusion had a protective effect when started 18 hours later (100). Although early studies in humans reported some positive results (53), controlled trials failed to show a significant improvement (17,23,75).
In summary, endogenous glucagon from the endocrine α-cells may have an action on the exocrine pancreas, but its mechanism of action and physiological importance is not clear. Supraphysiological administration can affect the exocrine pancreas, but its physiological significance is unclear. Glucagon affects insulin secretion and body metabolism and these could also secondarily affect the exocrine pancreas. Whether increased glucagon levels in diabetes could contribute to the reduced exocrine function reported in diabetics is currently unclear and worthy of further attention.
6. Tools for the Study of Glucagon
Detection and Measurement
Monoclonal and polyclonal antibodies against human, rat and mouse glucagon are commercially available for the detection of glucagon and pro-glucagon by immunoblotting, immunohistochemistry, immunofluorescence, and immunoprecipitation (Sigma, Abcam, Santa Cruz, Cell Signaling). RIAs and ELISAs are also commercially available for the measurement of glucagon in human, mouse and rat serum, plasma as well as tissue extracts and culture media (Millipore, Alpco, R&D Systems). A disadvantage in using a RIA or ELISA to measure glucagon levels in the serum or plasma of mice is the large volume of sample required to detect baseline levels (50-100 µl) in a concentration range of 20-400 pg/mL. Further modifications of these assays maybe required, but are not always sufficient, to accommodate smaller volumes. A new ELISA recently developed by Mercodia (Uppsala, Sweden) requires smaller volume (25 µl) and offers higher specificity of detection (5-414 pg/mL).
Cell Lines
A glucagon-secreting cell, alpha TC-1, is the only readily available cell line for in vitro approaches to study the regulation of glucagon synthesis and secretion and can be obtained from the American Type Culture Collection (ATCC; Manassas, VA). This cells line was extracted from adenomas developed in transgenic mice expressing the SV40 large T-antigen under the rat pre-proglucagon promoter and further differentiated in two clones (clones 6 and 9) which do not express insulin/proinsulin mRNA, somatostatin or pancreatic polypeptide (37).
InR1G9 is another glucagon-secreting cell line often referred to in the literature. The InR1G9 cell line is derived from hamster glucagonoma (20,21,78).
Animals
Loss of α-cells have been observed in transgenic animal models with transcription factor mis-expression resulting in either a loss of a-cells or diverting a-cell-fate into a different endocrine cell lineage (13,14,38,60,92). The loss of α-cell transcription factors Arx, Pax6 and Foxa2 results in a dramatic complete loss of a-cells and circulating glucagon levels (38,60,92). In contrast, ectopic expression of Pax4 drives endocrine precursor cells and mature a-cells to adapt a β-cell fate (14). Thorel and colleagues were able to ablate 98% of α-cells using an inducible system of diphtheria toxin-mediated cell deletion in adult mice (97). Animal models designed to block glucagon’s actions by a genetic or pharmacological inhibition of glucagon receptor and inhibiting active glucagon synthesis using prohormone convertase 2 knock-out mice, have been described (30,32,61,64).
7. References
- Adler G. Effect of glucagon on the secretory process in the rat exocrine pancreas. Cell Tiss Res 182: 193-204, 1977. PMID: 902302
- Asplin CM, Paquette TL, and Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 68: 314-318, 1981. PMID: 7019246
- Bandisode MS and Singh M. Amylase secretion from isolated pure acinar cells. Biochem Biophys Res Commun 129: 63-69, 1985. PMID: 2408620
- Bensley SH, Woerner CA. The effect of continuous intravenous injection of an extract of the alpha cells of the guinea pig pancreas on the intact guinea pig. Anat Rec 72 (Suppl):413, 1938.
- Biedzinski TM, Bataille D, Devaux MA, and Sarles H. The effect of oxyntomodulin (glucagon-37) and glucagon on exocrine pancreatic secretion in the conscious rat. Peptides 8: 967-972, 1987. PMID: 3441447
- Bonner-Weir S and Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31: 883-889, 1982. PMID: 6759221
- Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, and Gingerich R. Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 23: 302-308, 2001. PMID: 11590327
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, and Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103: 2334-2339, 2006. PMID: 16461897
- Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Kohler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, and Berggren PO. Glutamate is a positive autocrine signal for glucagon release. Cell Metabolism 7: 545-554, 2008. PMID: 18522835
- Cameron JM and Melrose AG. Changes in liver, pancreatic and stomach morphology following chronic glucaon administration in guinea-pigs. Br J Exp Path 43: 384-386, 1962. PMID: 13875946
- Chen L, Philippe J, and Unger RH. Glucagon responses of isolated alpha cells to glucose, insulin, somatostatin, and leptin. Endocrine Practice 17: 819-825, 2011. PMID: 13875946
- Clain JE, Barbezat GO, Waterworth MM, and Bank S. Glucagon inhibition of secretin and combined secretin and cholecystokinin stimulated pancreatic exocrine secretion in health and disease. Digestion 17:11-17, 1978. PMID: 627317
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, and Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes & Devlopment 17: 2591-2603, 2003. PMID: 14561778
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, and Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 138: 449-462, 2009. PMID: 14561778
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 45: 937-948, 2002. PMID: 12136392
- Danielsson Å. Effects of glucose, insulin and glucagon on amylase secretion from incubated mouse pancreas. Pflügers Arch 348:333-342, 1974. PMID:4857978
- Debas HT, Hancock RJ, Soon-Shiong O, Smythe HA, and Cassim MM. Glucagon therapy in acute pancreatitis: prospective randomized double-blind study. Can J Surg 23: 578-580, 1980. PMID: 6160901
- DiMagno EP, Go VWL, Summerskill. Intraluminal and postabsorptive effects of amino acids on pancreatic enzyme secretion. J Lab Clin Med 82:241-248, 1973. PMID: 4721379
- Dinneen S, Alzaid A, Turk D, and Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia 38: 337-343, 1995. PMID: 7758881
- Drucker DJ, Philippe J, and Mojsov S. Proglucagon gene expression and posttranslational processing in a hamster islet cell line. Endocrinology 123: 1861-1867, 1988. PMID: 3416818
- Dumonteil E, Ritz-Laser B, Magnan C, Grigorescu I, Ktorza A, and Philippe J. Chronic exposure to high glucose concentrations increases proglucagon messenger ribonucleic acid levels and glucagon release from InR1G9 cells. Endocrinology 140: 4644-4650, 1999. PMID: 10499521
- Dunphy JL, Taylor RG, and Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 141: 179-186, 1998. PMID: 9723898
- Dürr HK, Maroske D, Zelder O, Bode JC. Glucagon therapy in acute pancreatitis. Report of a double-blind trial. Gut 19:175-179, 1978. PMID: 344159
- Dyck WP, Rudick J, Hoexter B, and Janowitz HD. Influence of glucagon on pancreatic exocrine secretion. Gastroenterology 56: 531-537, 1969. PMID: 5766909
- Dyck WP, Texter EC, Lasater JM, and Hightower NC Jr. Influence of glucagon on pancreatic exocrine secretion in man. Gastroenterology 58: 532-539, 1970. PMID: 5438004
- Farah AE. Glucagon and the circulation. Pharmacol Rev 35: 181-217, 1983. PMID: 6318231
- Filippi BM, Abraham MA, Yue JT, and Lam TK. Insulin and glucagon signaling in the central nervous system. Reviews in Endocrine & Metabolic Disorders, 2013. PMID: 23959343
- Foa PP SL, Weinstein H, Berger S, Smith JA. Secretion of the hyperglycemic-glycogenolytic factor in normal dog. Am J Physiol: 32-36, 1952. PMID: 12985958
- Franklin I, Gromada J, Gjinovci A, Theander S, Wolheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808-1815, 2005. PMID:15919803
- Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, and Steiner DF. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem 276: 27197-27202, 2001. PMID: 11356850
- Gaisano HY, Macdonald PE, Vranic M. Glucagon secretion and signaling in the development of diabetes. Frontiers in Physiology 3:349, 2012. PMID:22969729
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, and Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100: 1438-1443, 2003. PMID: 12552113
- Gerich JE, Langlois M, Noacco C, Karam JH, and Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 182: 171-173, 1973. PMID: 4581053
- Gerich JE, Lorenzi M, Schneider V, Kwan CW, Karam JH, Guillemin R, and Forsham PH. Inhibition of pancreatic glucagon responses to arginine by somatostatin in normal man and in insulin-dependent diabetics. Diabetes 23: 876-880, 1974. PMID: 4430415
- Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, and Henquin JC. The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129: 2521-2529, 1991. PMID: 1682137
- Gromada J, Franklin I, and Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84-116, 2007. PMID: 17261637
- Hamaguchi K and Leiter EH. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes 39: 415-425, 1990. PMID: 2108069
- Hancock AS, Du A, Liu J, Miller M, and May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol 24: 1605-1614, 2010. PMID: 20592160
- Hansen LH, Abrahamsen N, and Nishimura E. Glucagon receptor mRNA distribution in rat tissues. Peptides 16: 1163-1166, 1995. PMID: 8532603
- Harada H, Kochi F, Hanafusa E, Kobayashi T, Oka H, Kimura I. Studies on the effect of glucagon on human pancreatic secretion by analysis of endoscopically obtained pure pancreatic juice. Gastroenterol Jpn 20: 28-36, 1985. PMID 4018495
- Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, and Jones PM. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58: 403-411, 2009. PMID: 18984743
- Heimberg H, De Vos A, Moens K, Quartier E, Bouwens L, Pipeleers D, Van Schaftingen E, Madsen O, and Schuit F. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc Natl Acad Sci USA 93: 7036-7041, 1996. PMID: 8692940
- Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 22:158-167, 1981. PMID: 6111521
- Horiguchi Y. Interaction of Secretin and Glucagon on Exocrine Pancreatic Secretion. Gastroenterology 14: 63-73, 1979. PMID: 446988
- Horiuchi A, Iwatsuki K, Ren, L, Kuroda T, Chiba S. Dual Actions of Glucagon: Direct Stimulation and Indirect Inhibition of Dog Pancreatic Secretion. Eur J Pharmacol 237: 23-30, 1993. PMID: 7689468
- Itoh H, Matsuyama T, Namba M, Watanbe N, Komatsu R, Kono N, Tarui S. Effect of Glucagon-(1-21)-Peptide on secretin-Stimulated Pancreatic Exocrine Secretion in Anesthetized Dogs. Life Sciences 44: 819-825, 1989. PMID: 2704290
- Iwatsuki K, Ono H, and Hashimoto K. Effects of glucagon on pancreatic secretion in the dog. Clin Exp Pharmacol Physiol 3: 59-65, 1976. PMID: 971553
- Jarett L. In vivo and in vitro effect of glucagon on DL-Leucine-1-C incorporation into protein of rat pancreas. Proc Soc Exp Biol Med 114: 550-554, 1963. PMID: 14115254
- Jarett L and Lacy PE. Effect of glucagon on the acinar portion of the pancreas. Endocrinology 70: 867-873, 1962. PMID: 14451306
- Jiang G and Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284: E671-678, 2003. PMID: 12626323
- Kash F, Woods JG, and Solomon T. Glucagon inhibition of cerulein-induced hypertrophy of the exocrine pancreas. Pancreas 3: 11-17, 1988. PMID: 3362838
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, and Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metabolism 9: 350-361, 2009. PMID: 19356716
- Knight MJ, Condon JR, Smith R. Possible use of glucagon in the treatment of pancreatitis. Br Med J 2:440-442, 1971. PMID:5576005
- Konturek S, Demitrescu T, Radecki T, Thor P, Pucher A. Effect of glucagon on Gastric and Pancreatic Secretion and Peptic Ulcer Formation in Cats. Digestive Diseases 19: 557-564, 1974. PMID: 4597820
- Konturek SJ, Tasler J, and Obtulowicz W. Characteristics of inhibition of pancreatic secretion by glucagon. Digestion 10: 138-149, 1974. PMID: 4841910
- Konturek SK, Tasler J, and Obtulowicz W. Effect of glucagon on food-induced gastrointestinal secretions. Digestion 8: 220-226, 1973. PMID: 4721701
- Lacy PE, Cardeza AF, Wilson WD. Electron microscopy of the rat pancreas. Effects of glucagon administration. Diabetes 8:36-44, 1959. PMID:13619489
- Lane MA. The cytological characters of the areas of Langerhans. Am J Anat: 409-422, 1907.
- Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Diabetologia 54:125-134, 2011. PMID:20938634
- Lee CS, Sund NJ, Behr R, Herrera PL, and Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Developmental biology 278: 484-495, 2005. PMID: 15680365
- Lee Y, Wang MY, Du XQ, Charron MJ, and Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60: 391-397, 2011. PMID: 21270251
- Lefebvre PJ. Glucagon and its family revisited. Diabetes Care 18: 715-730, 1995. PMID: 8586014
- Liu Z, Kim W, Chen Z, Shin YK, Carlson OD, Fiori JL, Xin L, Napora JK, Short R, Odetunde JO, Lao Q, and Egan JM. Insulin and glucagon regulate pancreatic alpha-cell proliferation. PloS one 6: e16096, 2011. PMID: 21283589
- Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, and Drucker DJ. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: evidence for a circulating alpha-cell growth factor. Diabetes 62: 1196-1205, 2013. PMID: 23160527
- Manabe T, Steer M. Experimental acute pancreatitis in mice. Protective effects of glucagon. Gastroenterology 76: 529-534, 1979. PMID: 428707
- Manabe T, Steer M. Effects of glucagon on pancreatic content and secretion of amylase in mice. Proc Soc Exp Biol Med 161: 538-542, 1979. PMID: 482288
- Maruyama H, Hisatomi A, Orci L, Grodsky GM, and Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74: 2296-2299, 1984. PMID: 6392344
- Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, and Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmcol Rev 55: 167-194, 2003. PMID: 12615957
- Miller RE, Chernish SM, Greenman GF, Maglinte DDT, Rosenak BD, Brunelle. Gastrointestinal response to minute doses of glucagon. Radiology 143:317-320. PMID:7071331
- Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, and Schuit F. Dual glucagon recognition by pancreatic beta-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 47: 66-72, 1998. PMID: 9421376
- Murlin JR, Clough HD, Gibbs CBF, Stokes AM. Aqueous extracts of the pancreas. 1. Influence on the carbohydrate metabolism of the depancreatized animals. J Biol Chem: 56:253-296, 1923. PMID:
- Murphy JJ and McGeeney KF. The effects of glucaon on the exocrine secretion of the perfused canine pancreas. Ir J Med Sci 143: 37-41, 1974. PMID: 4814741
- Nakajima S and Magee DF. Inhibition of exocrine pancreatic secretion by glucagon and D-glucose given intravenously. Can J Physiol Pharamcol 48: 299-305, 1970. PMID: 5422425
- Necheles H. Effects of glucagon on external secretion of the pancreas. Am J Physiol. 191:595-597, 1957. PMID: 13487787
- Olazabal A and Fuller R. Failure of glucagon in the treatment of alcoholic pancreatitis. Gastroenterology 74: 489-491, 1978. PMID: 344125
- Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, and Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology 146: 4861-4870, 2005. PMID: 16081632
- Pandol SJ, Sutliff VE, Jones SW, Charlton CG, O’Donohue TL, Gardner JD, and Jensen RT. Action of natural glucagon on pancreatic acini: due to contamination by previously undescribed secretagogues. Am J Physiol 245: G703-G710, 1983. PMID: 6195929
- Philippe J. Glucagon gene transcription is negatively regulated by insulin in a hamster islet cell line. J Clin Invest 84: 672-677, 1989. PMID: 2668337
- Reaven GM, Chen YD, Golay A, Swislocki AL, and Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64: 106-110, 1987. PMID: 3536980
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, and Smith PA. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341: 233-236, 1989. PMID: 2550826
- Salter JM, Davidson IWF, Best CH. The pathologic effects of large amounts of glucagon. Diabetes 6:248-252, 1957. PMID: 13427630
- Schinner S, Barthel A, Dellas C, Grzeskowiak R, Sharma SK, Oetjen E, Blume R, and Knepel W. Protein kinase B activity is sufficient to mimic the effect of insulin on glucagon gene transcription. J Biol Chem 280: 7369-7376, 2005. PMID: 15590659
- Shaw HM and Heath TJ. The effect of glucagon on the formation of pancreatic juice and bile in the rat. Can J Physiol Pharmacol 51: 1-5, 1973. PMID: 4692196
- Shen XX, Li HL, Pan L, Hong J, Xiao J, Hermansen K, Jeppesen PB, and Li GW. Glucotoxicity and alpha cell dysfunction: involvement of the PI3K/Akt pathway in glucose-induced insulin resistance in rat islets and clonal alphaTC1-6 cells. Endocrine Res 37: 12-24, 2012. PMID: 22007944
- Singer M, Tiscornia O, Mendes De Oliveiro, J, Demol P, Levesque D, and Sarles H. Effect of Glucagon on Canine Exocrine Pancreatic Secretion Stimulated by a Test Meal. Can J Physiol Pharmacol 56: 2481-2487, 1978. PMID: 638847
- Singh J and Adeghate E. Effects of islet hormones on nerve-mediated and acetylcholine-evoked secretory responses in the isolated pancreas of normal and diabetic rats. Int J Mol Med 1: 627-634, 1998. PMID: 9852277
- Singh J, Adeghate E, Salido GM, Pariente JA, Yago MD, and Juma LOM. Interaction of islet hormones with cholecystokinin octapeptide-evoked secretory responses in the isolated pancreas of normal and diabetic rats. Exp Physiol 84: 299-318, 1999. PMID: 10226172
- Singh M. Amylase release from dissociated mouse pancreatic acinar cells stimulated by glucagon: effect of membrane stabilizers. J Physiol 309: 81-91, 1980. PMID: 6166745
- Singh M. Effect of glucagon on digestive enzyme synthesis, transport and secretion in mouse pancreatic acinar cells. J Physiol 306: 307-322, 1980. PMID: 6162027
- Singh M. Role of cyclic adenosine monophosphate in amylase release from dissociated rat pancreatic acini. J Physiol 331: 547-555, 1982. PMID: 6185668
- Sommer H and Kasper H. Effect of acetylcholine, gastrin, and glucagon alone and in combination with secretin and cholecystokinin on the secretion of the isolated perfused rat pancreas. Res Exp Med (Berl) 179: 239-247, 1981. PMID: 7323454
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, and Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 387: 406-409, 1997. PMID: 9163426
- Stagner JI, Samols E, and Marks V. The anterograde and retrograde infusion of glucagon antibodies suggests that A cells are vascularly perfused before D cells within the rat islet. Diabetologia 32: 203-206, 1989. PMID: 2568960
- Staritz M. Pharmacology of the sphinter of Oddi. Endoscopy 20 Suppl 1:171-174, 1988. PMID: 3049055
- Sutherland EW and De Duve C. A glycogenolytic factor from pancreas. Fed Proc 7: 195, 1948. PMID: 18860120
- Sutherland EW and De Duve C. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem 175: 663-674, 1948. PMID: 18880761
- Thorel F, Damond N, Chera S, Wiederkehr A, Thorens B, Meda P, Wollheim CB, and Herrera PL. Normal glucagon signaling and beta-cell function after near-total alpha-cell ablation in adult mice. Diabetes 60: 2872-2882, 2011. PMID: 21926270
- Von Schonfeld J, Muller K. The islet-acinar axis of the pancreas: Is there a role for glucagon or glucagon-like peptide? Experientia 50: 442-446, 1994. PMID: 7515009
- Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obesity and Metabolism 13 Suppl 1:95-105, 2011. PMID:21824262
- Waterworth MW, Barbezat, Hickman R, and Terblanche J. A controlled trial of glucagon in experimental pancreatitis. Br J. Surg 63:617-620. PMID: 953466
- Weir GC, Atkins RF, and Martin DB. Glucagon secretion from the perfused rat pancreas following acute and chronic streptozotocin. Metabolism: Clin Exp 25: 1519-1521, 1976. PMID: 135913
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, and Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes 53: 1038-1045, 2004. PMID: 15047619
- Wierup N, Yang S, McEvilly RJ, Mulder H, and Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52: 301-310, 2004. PMID: 14966197
- Wizemann V, Weppler P, and Mahrt R. Effect of glucagon and insulin on the isolated exocrine pancreas. Digestion 11: 432-435, 1974. PMID: 15367896
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, and Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell metabolism 3: 47-58, 2006. PMID: 16399504
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88: 2300-2308, 2003. PMID: 12727989
- Zhang Y, Zhang Y, Bone RN, Cui W, Peng JB, Siegal GP, Wang H, and Wu H. Regeneration of pancreatic non-beta endocrine cells in adult mice following a single diabetes-inducing dose of streptozotocin. PloS one 7: e36675, 2012. PMID: 22586489


