Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2012.7
| Attachment | Size |
|---|---|
| 191.15 KB |
Gene Symbol: Carhsp1
Alternate Names: CRHSP24, CARHSP1
1. General Information
CRHSP-24, also known as CARHSP1, is named for its properties as a calcium regulated, heat stable protein of 24 kDa and was identified in a phosphoproteomic screen of pancreatic acinar proteins. Two dimensional gels were used to separate proteins after 32P metabolic labeling and CRHSP-24 showed secretagogue mediated dephosphorylation (4,5,24). The dephosphorylation was later shown to be inhibited by cyclosporine A and FK506, inhibitors of calcineurin (link to Calcineurin molecule page) and could be mimicked by adding constitutively active calcineurin to acinar cell lysate (12). The protein was then purified from rat pancreas making use of its heat stable property, sequenced and characterized as a 147 amino acid, proline rich protein (13). The protein is broadly present in various tissues with high levels in testis, liver, pancreas and other exocrine glands. Its phosphorylation is entirely on Ser residues.
Immunodetection of CRHSP-24 in pancreatic acini showed multiple phosphorylated forms separable by 1D IEF consistent with four regulated phosphorylation sites. A mutation study of CRHSP-24 in which all fourteen serine residues were mutated established the phosphorylation sites as Ser-30, -32, -41, and 52 (15). Of these, Ser-30 and -32 were specifically dephosphorylated in response to an increased cellular calcium and inhibited by CsA indicating that calcineurin is targeting these sites. CRHSP-24 is also partially dephosphorylated in response to cAMP (24) and this was shown to be stimulated by a phosphatase inhibited by calyculin A and okadaic acid, namely a PP2A or PP4 (21). This is most likely at Ser-52 as this site was not sensitive to calcium. Less is known of the kinases phosphorylating CRHSP-24 under physiological conditions. Auld et al (2) identified CRHSP-24 as a PKB (Akt) substrate and the site of phosphorylation as Ser-52. IGF-1 stimulated this phosphorylation in HEK-293 cells which could be inhibited with wortmannin, a PI3K inhibitor. Bacterially produced CRHSP-24 was also shown to be phosphorylated in vitro by DYRK2, a constitutively active proline directed kinase on Ser-30, -32, and -41 (6,25). In serum fed cells, both Ser 41 and -52 were phosphorylated as identified by mass spectrometry (2). Subsequently, phosphorylation of all four sites plus Ser-2 phosphorylation were identified by LC-MS/MS in C2C12 myoblasts stimulated to differentiate (18).
CRHSP-24 was found to be homologous to a brain specific paralog known as PIPPin which had been identified in a screen for RNA binding proteins (3,7,16). Both CRHSP-24 and PIPPin possess RNA binding domains and a central cold shock domain (CSD). The overall domain structure of CRHSP-24 is shown in Fig. 1 and a detailed comparison of the sequence of these proteins from mammals to Drosophila and Schistosomes has been presented (15).
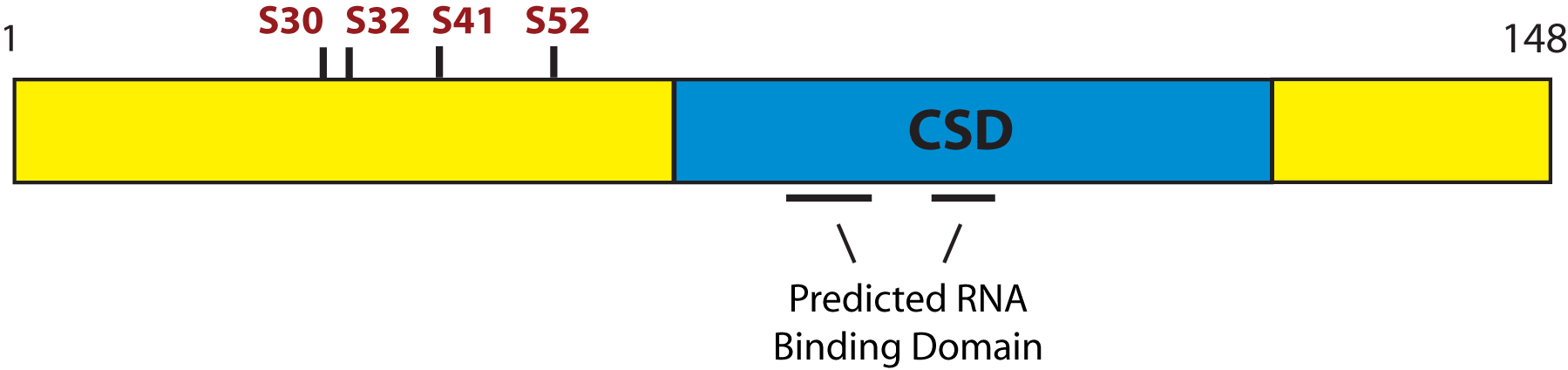
Fig. 1. Domain structure of mouse CRHSP-24. The Cold Shock Domain (CSD) is shown in blue and the phosphorylation sites in red.
The crystal structure of CRHSP-24 was recently reported and shown to contain an N terminal alpha helix and a central β-barrel nearly identical to a bacterial CSD (14). In this study CRHSP-24 was shown to form dimers and tetramers in solution and also to bind to a RNA sequence from the 3’ untranslated region of Histone H3.3, a mRNA previously shown to bind PIPPin (16). While CRHSP-24 was primarily cytosolic in control HeLa cells (similar to prior localization in pancreatic acini), upon exposure to oxidizing conditions it concentrated in stress granules and processing bodies (P-bodies) (14). P-bodies and stress granules are distinct structures but act together in response to stress conditions to sequester untranslated mRNA (1). CRHSP-24 was also found to bind the 3’ region of TNF-α mRNA, to inhibit its degredation, and to localize in P-bodies and exosome granules (17). In this study calcineurin activity was required for the function of mRNA stability. Taken together, these studies show that CRHSP-24 functions at the site of mRNA degradation and is not involved directly with translation.
Several other potential functions of CRHSP-24 have been identified. CRHSP-24 showed increased phosphorylation during HeLa cell adhesion and spreading on a collagen coated surface suggesting that it might play a role in the response to integrin binding (8). CRHSP-24 was also identified in a complex with STYX, an inactive dual specificity phosphatase, present in spermatids (23). Reduction of CRHSP-24 levels by siRNA in S. japonicum altered parasite morphology and caused parasite death (26). Finally in mammalian liver CARHSP1 (CRHSP-24) inhibited hepatic glucogenic gene expression by repression of PPARα (10). Possibly related to this, Carhsp1 was identified in a screen of genes upregulated in diabetic retinopathy (11). Whether these other actions are mediated by effects on mRNA stability or represent other actions of the molecule will require further study.
2. CRHSP-24 in The Exocrine Pancreas
CRHSP-24 is abundantly present in pancreatic acini and has been identified as a regulated phosphoprotein in rat, mouse and guinea pig isolated acini as outlined above (4,5,21,). However, little is known of its function in acinar cells. Because of the property of CRHSP-24 as an RNA binding protein it seems likely that it could be regulating protein translation. In support of this possibility, feeding of fasted mice increased the rate of pancreatic protein synthesis (which is made up primarily of digestive enzymes) by a translational mechanism (19). Calcineurin activity was shown to be required for both translational control of protein synthesis in rat isolated acini (20) and for pancreatic adaptive growth in response to sustained endogenous CCK release in response to feeding trypsin inhibitor (22). Whether and how CRHSP-24 is involved in these processes, however, is unknown at present. CRHSP-24 dephosphorylation has been used as an assay for calcineurin activation in response to CCK (20) and to show that rapamycin, an mTOR inhibitor did not inhibit calcineurin (9).
3. Tools to Study CRHSP-24
a. cDNA clones
Human and rat CRHSP-24 clones in pcDNA3.1 and pGEX have been reported in multiple papers referred to in this entry (2,10,14,15). Some are a GFP fusion protein or with an epitope tag.
b. Antibodies
Multiple antibodies to CRHSP-24 have been reported. Our lab generated a rabbit antisera to GST-CRHSP-24 fusion protein which worked for both immunoblotting and IHC (13,15,23). Auld et al (2) generated a sheep Ab against the full length GST fusion protein which was passed through a GST column to remove antiGST antibodies. Commercially antibodies include rabbit and chicken antibodies from AbCam and goat polyclonal and mouse monoclonal from Santa Cruz; the latter was used for IHC as well as immunoblotting (14). These latter antibodies are found in the catalogs as against CARHSP1. An antibody specific for CRHSP-24 phosphorylated at Ser-52 has also been generated (2).
c. Viral Vectors
An adenoviral vector to express human CRHSP-24 was constructed and used to overexpress the protein in mouse liver or hepatic cells in culture (10).
d. siRNA
A 21-mer siRNA from Santa Cruz has been reported to knock down CRHSP-24 protein (14) and a siRNA from Ambion was used in (10). siRNAs against S. japonicum have also been used (26).
4. References
- Anderson P, Kedersha N. RNA granules. J Cell Biol., 172:803-808, 2006. PMID: 16520386
- Auld GC, Campbell DG, Morrice N, Cohen P. Identification of calcium-regulated heat-stable protein of 24 kDa (CRHSP24) as a physiological substrate for PKB and RSK using KESTREL. Biochem J., 389: 775-783, 2005. PMID: 15910284
- Bono E, Compagno V, Proia P, Raimondi L, Schiera G, Favaloro V, Campo V, Donatelli M, Di Liegro I. Thyroid hormones induce sumoylation of the cold shock domain-containing protein PIPPin in developing rat brain and in cultured neurons. Endocrinology, 148: 252-257, 2007. PMID: 17053029
- Burnham DB, Munowitz P, Hootman SR, Williams JA. Regulation of protein phosphorylation in pancreatic acini. Distinct effects of Ca2+ ionophore A23187 and 12-O-tetradecanoylphorbol 13-acetate. Biochem J., 235: 125-131, 1986. PMID: 2427068
- Burnham DB, Sung CK, Munowitz P, Williams JA. Regulation of protein phosphorylation in pancreatic acini by cyclic AMP-mediated secretagogues: interaction with carbamylcholine. Biochem Biophys Acta., 969: 33-39, 1988. PMID: 2450590
- Campbell LE, Proud CG. Differing substrate specificities of members of the DYRK family of arginine-directed protein kinases. FEBS Lett., 510: 31-36, 2002. PMID: 11755526
- Castiglia D, Scaturro M, Nastasi T, Cestelli A, Di Liegro I. PIPPin, a putative RNA-binding protein specifically expressed in the rat brain. Biochem Biophys Res Commun., 218: 390-394, 1996. PMID: 8573167
- Chen Y, Lu B, Yang Q, Fearns C, Yates III JR, Lee JD. Combined integrin phosphoproteomic analyses and small interfering RNA-based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res., 69: 3713-3720, 2009. PMID: 19351860
- Crozier SJ, Sans MD, Guo L, D’Alecy LG, Williams JA. Activation of the mTOR signaling pathway is required for pancreatic growth in protease-inhibitor-fed mice. J Physiol., 573: 775-786, 2006. PMID: 16613881
- Fan Y, Guo Y, Hamblin M, Chang L, Zhang J, Chen YE. Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor α. J Biol Chem., 286: 40584-40594, 2011. PMID: 21990353
- Freeman WM, Bixler GV, Brucklacher RM, Lin C-M, Patel KM, VanGuilder HD, LaNoue KF, Kimball SR, Barber AJ, Antonetti DA, Gardner TW, Bronson SK. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics J. 10:385-395, 2010. PMID: 19997081
- Groblewski GE, Wagner ACC, Williams JA. Cyclosporin A inhibits Ca2+/Calmodulin-dependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem., 269: 15111-15117, 1994. PMID: 7515049
- Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem., 273: 22738-22744, 1998. PMID: 9712905
- Hou H, Wang F, Zhang W, Wang D, Li X, Bartlam M, Yao X, Rao Z. Structure-functional analyses of CRHSP-24 plasticity and dynamics in oxidative stress response. J Biol Chem., 286: 9623-9635, 2011. PMID: 21177848
- Lee S, Wishart MJ, Williams JA. Identification of calcineurin regulated phophorylation sites on CRHSP-24. Biochem Biophys Res Commun., 385: 413-417, 2009. PMID: 19477163
- Nastasi T, Scaturro M, Bellafiore M, Raimondi L, Beccari S, Cestelli A, Di Liegro I. PIPPin is a brain-specific protein that contains a cold-shock domain and binds specifically to H1° and H3.3 mRNAs. J Biol Chem., 274: 24087-24093, 1999. PMID: 10446180
- Pfeiffer JR, McAvoy BL, Fecteau RE, Deleault KM, Brooks SA. CARHSP1 is required for effective tumor necrosis factor alpha mRNA stabilization and localizes to processing bodies and exosomes. Mol Cell Biol., 31: 277-286, 2011. PMID: 21078874
- Puente LG, Voisin S, Lee REC, Megeney LA. Reconstructing the regulatory kinase pathways of myogenesis from phosphopeptide data. Mol Cell Proteomics., 5: 2244-2251, 2006. PMID: 16971385
- Sans MD, Lee SH, D’Alecy LG, Williams JA. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol., 287: G667-G675, 2004. PMID: 15117679
- Sans MD, Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am J Physiol Cell Physiol., 287: C310-C319, 2004. PMID: 15044154
- Schäfer C, Steffen H, Krzykowski KJ, Göke B, Groblewski GE. CRHSP-24 phosphorylation is regulated by multiple signaling pathways in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol., 285: G726-G734, 2003. PMID: 12801884
- Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol., 286: G784-G790, 2004. PMID: 1468438
- Wishart MJ, Dixon JE. The archetype STYX/dead-phosphatase complexes with a spermatid mRNA-binding protein and is essential for normal sperm production. Proc Natl Acad Sci USA., 99: 2112-2117, 2002. PMID: 11842224
- Wishart MJ, Groblewski G, Göke BJ, Wagner ACC, Williams JA. Secretagogue regulation of pancreatic acinar cell protein phosphorylation shown by large-scale 2D-PAGE. Am J Physiol., 267: G676-G686, 1994. PMID: 7943332
- Woods YL, Cohen P, Becker W, Jakes R, Goedert M, Wang X, Proud CG. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bε at Ser539 and the microtublule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J. 355:609-615, 2001). PMID: 11311120
- Zou X, Jin Y, Liu P, Wu Q, Liu J, Lin J. RNAi silencing of calcium-regulated heat-stable protein of 24 kDa in Schistosoma japonicum affects parasite growth. Parasitol Res., 108: 567-572, 2011. PMID: 21085993

