Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2018.18
| Attachment | Size |
|---|---|
| 621.68 KB |
Gene Symbol: CCK
Abstract
In 1928, Ivy and Oldberg discovered that intestinal extracts prepared after instilling weak acid or fats into the proximal duodenum, elicited gallbladder contraction in dogs, cats, and guinea pigs (33). Based on this biological property, the hormone was named cholecystokinin (CCK). In addition to gallbladder contraction, CCK was later shown to stimulate pancreatic secretion (55) and to delay gastric emptying by its effect on the lower esophageal sphincter (80). CCK was the first hormone shown to influence satiety and cause reduction in food intake (23). Due to this discovery and the implications of CCK’s therapeutic potential for eating disorders, considerable attention has focused on the study of this hormone.
In the gastrointestinal tract, CCK is secreted by discrete enteroendocrine cells (EECs) which contain intermediate-size secretory granules (I cells) (95). CCK-producing cells are primarily located in the proximal small intestine (duodenum and jejunum, Figure 1), and their numbers decrease significantly towards the distal end (ileum and colon) (Figure 2). CCK cells are often flask-shaped with the narrow apical edge facing the gut lumen. The basolateral membrane often contains one or more basal process(es) named neuropods that run alongside or project into the lamina propria (6, 10). Neuropods contain neuronal markers and have been shown to interact with enteric nerves suggesting that in addition to secretion to the blood, CCK can be released directly adjacent to enteric nerves (Figure 3). CCK immunoreactivity is abundant in the pyloric region of mouse stomach (45), cerebral cortex, dopaminergic neurons projecting to the limbic forebrain and ventromedial hypothalamus, peripheral nerves of the gastrointestinal tract, celiac plexus, and vagus nerve (2, 48). CCK has been shown to function both as a hormone and a neurotransmitter and belongs to the ‘brain-gut’ family of peptides. In addition to intestinal and neuronal expression, CCK is also expressed in other tissues such as the urogenital tract and heart (78). The structure of CCK and its function, pertaining to its role in the gastrointestinal tract, is discussed in this review.
Figure 1: Transverse section of mouse duodenum showing CCK cells (green). Nuclei are stained with DAPI (blue).
Figure 2:
The number of CCK cells is highest in the proximal small intestine of the mouse and decreases exponentially towards the distal end (ileum). CCK antibody (8) used for immunostaining sections did not react with gastrin. Number of cells in 5 sections spread over 1 inch in length were counted (R. Chandra, unpublished data).
Figure 3: Transverse section of CCK-EGFP mouse duodenum showing EGFP positive CCK cells (green) and enteric nerves immunostained for pan-neuronal marker PGP9.5 (red). Two CCK cells from the left panel, Cells A and B, are shown at higher magnification on the right. Cell A has three short whisker-like neuropods and its basolateral surface is in contact with enteric nerves. Cell B has 2 thin neuropods (arrows). The longer of the two neuropods terminates in a bulb and is in contact with a nerve.
1. General
CCK is present in all vertebrates from fish to mammals. A CCK-like peptide has been found in the protochordate Ciona intestinalis, suggesting that the CCK/gastrin family probably arose 500 million years ago (37). Based on the phylogeny of CCK and gastrin genes in protochordates versus cartilaginous fish such as Squalus acanthias, and amphibians, it is proposed that gene duplication occurred 350 million years ago during the appearance of cartilaginous fish (37, 38). In humans, the CCK gene is present on chromosome 3, spans 7 kb, and consists of three exons, the first of which is noncoding (87, 88). The mouse Cck gene is similar in structure to the human gene and is present on chromosome 9 in a syntenic cluster (21).
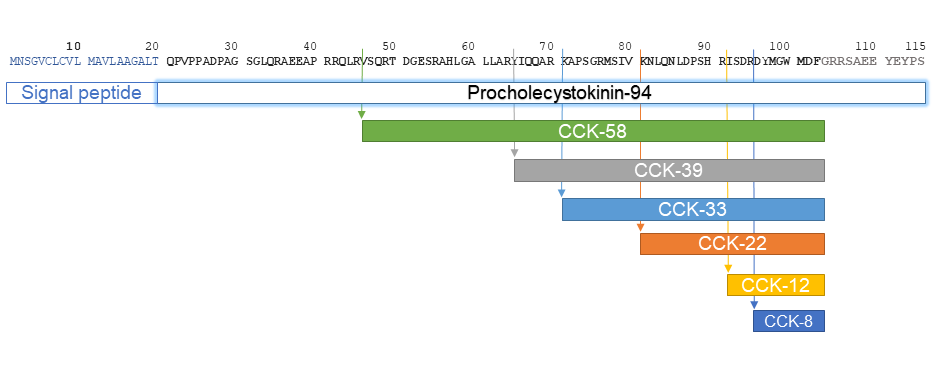
CCK polypeptides of various lengths have been described in the literature (Figure 4). Although there are four known transcripts of the human CCK gene, only a single preprocholecystokinin polypeptide of 115 amino acids is synthesized. After proteolytic excision of the signal peptide by signal peptidase, procholecystokinin of 94 amino acids is generated. This is again cleaved on both the N (24 amino acids) and C (12 amino acids) termini by endopeptidase and proprotein convertase 1 respectively, to generate a mid-section polypeptide of 58 amino acids known as CCK-58, which is the largest known circulating form of the hormone (19). It contains a carboxyl-amidated phenylalanine and O-sulfated tyrosine residue, which is responsible for increasing its biological activity by approximately 100-fold (18, 76, 77). CCK-58 undergoes subsequent endopeptidase cleavage at single or double basic residues to generate shorter peptides, CCK-39, CCK- 33, CCK-22, CCK-12 and CCK-8 (3, 84). CCK-8 is the smallest peptide which exhibits complete biological activity and is used most often in experiments for assessing CCK function. The five C-terminal residues of CCK are identical to gastrin, and as a result these two hormones display some functional similarities. This sequence identity complicated the measurement of CCK in the blood, as many antibodies against CCK-8 cross react with gastrin which is present at much higher concentrations in the blood (5).
Figure 4: Amino acid structure of the human CCK precursos and the different forms of CCK produced by processing.
Regulation of CCK secretion
CCK is released from EECs in response to entry of food into the duodenum. Plasma levels of CCK increase from basal levels of 0.5-1 pM to peak levels of 5-15 pM within a few minutes of food ingestion. In rodents, peak plasm levels are usually attained within 20 minutes of oral gavage. In humans, postprandial levels remain elevated for 3-5 hours until food empties from the stomach into the duodenum (57). Therefore, gastric emptying affects CCK secretion. Plasma CCK levels decline once food passes from the proximal small intestine. The half-life of CCK in the plasma is very short; in dogs the half-life of CCK-58 was 4.4 ± 0.6 minutes and that of CCK-8 was shown to be 1.3 ± 0.1 minutes (32). CCK is cleared from the circulation as it passes through the liver and by neutral endopeptidases in capillary endothelial cells (71).
CCK secretion is stimulated by ingested fats, proteins, and amino acids, whereas carbohydrates such as glucose cause only a brief, transient increase in circulating CCK levels (57).
The apical surface of CCK-producing cells is exposed to the intestinal lumen and receptors, located on the apical surface, can be stimulated by food molecules present in the lumen. Aromatic L-amino acids such as phenylalanine and tryptophan (but not non-aromatic amino acids such as alanine) stimulate CCK release through a Ca2+-dependent mechanism mediated by the calcium-sensing receptor (CaSR) (61, 94) while L-phenylalanine, L-leucine, and L-glutamic acid mediate CCK release through umami taste receptors T1R1 – T1R3 (14). In addition to amino acids, medium to long chain fatty acids (C12 and longer) also stimulate CCK release (65). The long chain fatty acid receptor GPR40 also known as free fatty acid receptor 1 (FFAR1), mediates some fatty acid-dependent CCK secretion (62). Fat meditated CCK-stimulation was completely eliminated in immunoglobulin-like domain containing receptor 1 (ILDR1) knockout mice suggesting that ILDR1 plays a role in CCK release (13). ILDR1-mediated CCK release occurred only in the presence of both high density lipoprotein (HDL) and fatty acids, suggesting a novel pathway in which uptake of HDL and/or fatty acid from the basolateral membrane could play an important role in CCK release.
Evidence is accumulating that cell surface receptors linked to hormone secretion may be located on the basolateral surface of the CCK cell. ILDR1-mediated CCK release requires both fatty acids and HDL which are most likely secreted onto the basolateral surface of the intestinal epithelium suggesting that CCK cells respond to absorbed nutrients (13). In addition, bile acid receptors have also been localized to the basolateral surface of EECs (8).
CCK secretion is under the control of negative feedback regulation by pancreatic proteases and bile acids (25, 56, 70). In most species, including humans, it has been shown that food-stimulated CCK secretion is suppressed by release of pancreatic proteases (31, 34, 54). This effect appears to be mediated by an endogenous protease-sensitive CCK-releasing peptide in the intestinal lumen (30, 50, 85). In addition to proteases, bile acids in the intestine affect CCK secretion (26, 55). In rats, luminal administration of taurocholate inhibited pancreatic enzyme secretion as well as CCK (90). In humans, single bile acids did not cause a decrease in CCK release, however, under conditions in which endogenous release of bile acid was inhibited by the CCK1 receptor antagonist loxiglumide, addition of a mixture of bile acids to a test meal prevented the increase in CCK release suggesting that bile acids play an important role in downregulating CCK secretion (43).
CCK released from EECs can act locally via a paracrine mechanism or enter the enteric blood stream and exert effects on distant target organs through hormonal mechanisms. There is evidence for neural activation of vagal afferents in the intestinal mucosa which express CCK1 receptors and terminate in the lamina propria (74). Although the effect of CCK on the vagus nerve was believed to be paracrine or hormonal action, recently, CCK cells have been shown to be in direct contact with neurons (7, 12, 53) and this connection may provide a direct neural link between the gut and brain.
Cholecystokinin Receptors
The action of CCK on tissues is mediated by two G protein-coupled receptors, CCK1 and CCK2, formerly known as CCK-A (for alimentary) and CCK-B (for brain) (17). CCK1 receptors are mainly located in the gastrointestinal tract, myenteric plexus, and vagal afferents and bind sulfated CCK with 1000-fold higher affinity than gastrin or non-sulfated CCK (16). CCK2 receptors are present in the stomach and the brain and have similar affinity for sulfated or non-sulfated CCK and for gastrin; hence this receptor is also known as the gastrin receptor. Development of receptor knockout mice have demonstrated that CCK1 receptors are important in regulation of CCK-mediated satiety responses (44) while CCK2 receptors are primarily involved in maintaining gastric morphology and acid secretion (47). A double CCK1/CCK2 knockout mouse displayed brain development abnormalities (67).
2. Actions of CCK
CCK Induces Gallbladder Contraction
CCK mediates bile release into the intestine by the dual action of stimulating gallbladder contraction and relaxing the sphincter of Oddi which allows bile to flow into the duodenum. In humans, infusion of CCK-8, decreased gallbladder volume by 80% and increased bilirubin output by 8 to 10-fold (82). These effects are mediated by CCK1 receptors that are located on both the smooth muscle layer of the gallbladder as well as cholinergic nerve terminals (81). The CCK1 receptor antagonist loxiglumide blocked bile release and CCK1 receptor knockout mice showed increased gallbladder volumes with enhanced susceptibility for gallstone formation compared to wild type mice (93).
CCK Stimulates Exocrine Pancreatic Secretion
Along with gallbladder contraction, the effects of CCK on pancreatic secretion were demonstrated in the first half of the 20th century when this hormone was also known as pancreozymin (29). CCK is one of the most important stimulants of pancreatic secretion (11). Physiological levels of exogenous CCK-33 administered to humans induced a trypsin output profile similar to that seen after a standardized test meal (40). The effect of CCK on human pancreatic enzyme output was partially blocked by the CCK1 receptor antagonist loxiglumide and completely inhibited by atropine suggesting that cholinergic activation is the major mechanism of CCK action (1, 22). In support of this finding, human acinar cells did not respond to CCK agonists and were shown to lack CCK1 and CCK2 receptors even though adenoviral mediated expression of CCK receptors on human acinar cells resulted in stimulation with CCK agonists (35). In contrast to humans, pancreatic secretion in rodents is mediated by direct activation of CCK1 receptors located on acinar cells as well as on vagal afferents (49, 83, 92).
CCK Stimulates Pancreatic Ductal Secretion
In addition to stimulation of protein by acinar cells, CCK promotes bicarbonate and fluid secretion from pancreatic ductal cells. Intraduodenal administration of corn oil in dogs led to elevated CCK levels along with protein and bicarbonate secretion (36). Secretion of bicarbonate and fluid was dependent on activation of CCK1 receptors, but not CCK2 receptors, and could be mimicked by a CCK1 receptor agonist (86).
Trophic effect of CCK on the pancreas
Several studies have demonstrated that CCK can increase pancreatic size (96). Exogenous administration of CCK increased pancreatic mass in hamsters and rats (27, 28, 68). In the rat, CCK1 receptor but not CCK2 receptor agonists increased pancreatic mass by increasing the number of cells comprising the exocrine pancreas (72). Since the human pancreas lacks CCK1 receptors, administration of ximelagatran, which has protease inhibitor activity and stimulates pancreatic growth in rats, did not have a significant effect on pancreatic growth in humans (56). Despite the lack of hypertrophic effects observed in human studies, it was recently shown that pancreatic atrophy resulting from total parenteral nutrition (TPN) could be reversed by CCK in rodents. When sulfated CCK-8 was infused in rats on TPN or an oral food diet, an increase in pancreatic mass was observed compared to non-CCK infused rats. The increase in pancreatic mass was somewhat less in rats on TPN suggesting that other factors may be involved in atrophy of the pancreas during TPN (98).
CCK Delays Gastric Emptying
CCK has been shown to have a pronounced effect in delaying gastric emptying in fish, rodents, dogs and humans (15, 69) by both relaxation of the proximal stomach and contraction of the pylorus (99). Administration of physiological levels of CCK-8 delayed gastric emptying in humans (52). In addition, this effect was dose-dependent and blocked by the CCK1 receptor antagonist loxiglumide (42, 52). Both vagal and splanchnic nerve pathways mediate the effect of CCK on gastric relaxation. In rats, bilateral cervical vagotomy partially reduced CCK-8-induced gastric relaxation but this response was completely eliminated when vagotomy was coupled with splanchnic nerve section (75).
CCK Induces Satiety
A seminal paper published in 1973 showed that administration of exogenous CCK-8 reduced food intake in rats (24). Similar effects have been noticed in a number of species including humans, where CCK-8 or CCK-33 infusion limited meal size and frequency (41, 59). CCK mediates satiety through its effects on CCK1 receptors located on vagal afferent nerves which provide negative feedback to the dorsal hind brain limiting food intake. The CCK1 receptor antagonist devazepide reduced the effects of CCK on satiety (4, 73) and increased hunger in humans (97). Moreover, Otsuka Long Evans Tokushima Fatty (OLETF) rats which lack CCK1 receptors (due to a spontaneous deletion of the promoter and exons 1 and 2 of the CCK1 receptor gene) were insensitive to reduction of feeding after administration of exogenous CCK (66). However, a CCK1 receptor specific agonist, GI191771X, which delayed gastric emptying, did not cause reduction in body weight of obese patients (BMI ≥30 or ≥27 kg/m2) in a 24‐week double‐blind trial, suggesting that regulation of this pathway by CCK was insufficient for controlling obesity (9, 39).
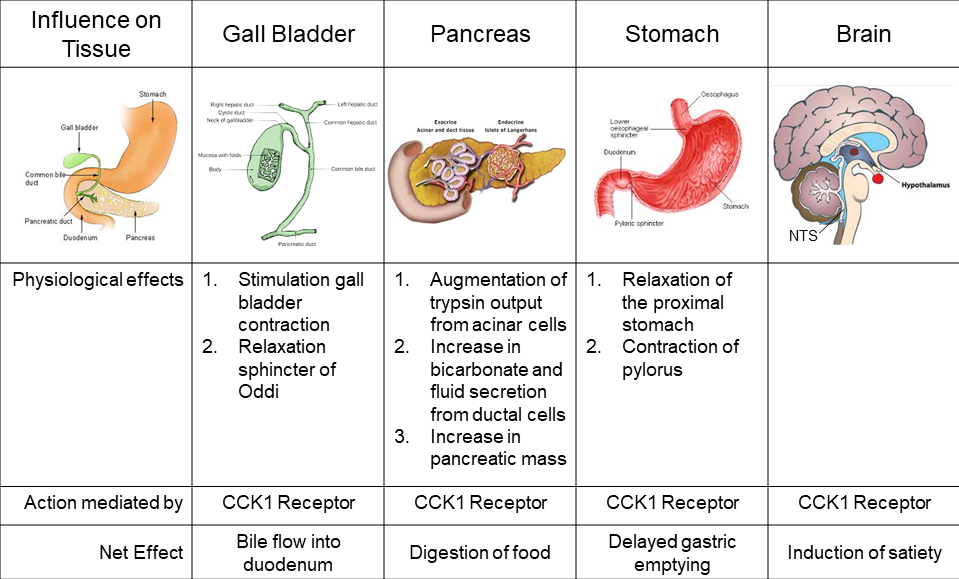
Figure 5: Physiological targets for CCK action.
In summary, CCK plays an important role in the regulation of postprandial gallbladder contraction, pancreatic secretion (enzyme, bicarbonate and fluid), as well as gastric emptying which optimizes the lumenal environment (pH) and regulates digestion of food in the gastrointestinal tract (Figure 5).
3. Tools for study of CCK
a. Peptide:
CCK-8 (DYMGWMDF-NH2) peptide retains full biological activity and is available in powder form from several vendors (Anaspec, Sigma Chemical Company, Tocris). Biologically active CCK-8 is sulfated on its tyrosine residue. De-sulfated CCK-8 peptide is often used as a control. Longer forms of CCK can be purchased as recombinant proteins.
b. Antibodies:
Antibodies that detect CCK on western blots or in tissues are available from numerous sources (Abcam Cat# ab134713, Sigma Chemical Company Cat# C2581, LSBio Cat# LS C293314). In our lab we generated a rabbit polyclonal to amino acids 19-36 of human CCK and affinity purified the antiserum over a peptide column (10). It should be noted that antibodies generated against CCK-8 peptide may also detect gastrin due to sequence identity of the last four amino acids.
c. cDNAs:
Human and rodent codon-optimized CCK cDNAs for expression in E. coli and mammalian cells are commercially available. Myc-tagged CCK cDNAs are also available.
d. Viral vectors:
Lentiviral particles of CCK tagged with myc or EGFP are available. Adenoviruses containing either the human or rodent CCK gene can also be purchased.
e. Assay:
ELISA kits with sensitivity in the range of 10 -1000 pg/mL are available from several vendors to measure CCK concentration. Kits from the following manufacturers are cited in literature: RayBiotech (100), Cloud-Clone Corp. (46), Phoenix Pharmaceuticals (20, 89). In addition, radioimmunoassays (RIA) are also used to quantitate CCK (60, 64, 91). In our laboratory we routinely use a CCK bioassay to measure CCK concentrations in human or rodent plasma samples (51, 58, 79). For the CCK bioassay, trunk blood is collected from three mice (1 ml total serum) per data point.
f. Mouse models:
CCK knockout mice (expressing lacZ reporter in cells where CCK is knocked out) and transgenic mice expressing EGFP in CCK cells are available from Jackson Labs and Mutant Mouse Resource and Research Centers (MMRRC) respectively. These mice have been characterized in various publications in the literature (10, 63).
g. Clinical Testing:
CCK-8 intravenous bolus injections (Sincalide 0.02 mcg/kg) are given to patients to perform clinical assessments: 1) Measurement of gallbladder contractility prior to cholecystectomy (https://clinicaltrials.gov/ct2/show/record/NCT02748525); 2) Evaluation of the composition of pancreatic secretion; and 3) Diagnosis of intestinal disorders by barium sulfate imaging; administration of CCK-8 accelerates the transit of barium through the GI tract. (https://www.rxlist.com/kinevac-drug.htm#description).
4. References:
- Adler G, Beglinger C, Braun U, Reinshagen M, Koop I, Schafmayer A, Rovati L, and Arnold R. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology 100: 537-543, 1991. PMID: 1702077
- Beinfeld MC. Cholecystokinin in the central nervous system: A minireview. Neuropeptides 3: 411-427, 1983, ; PMID: 6141537
- Beinfeld MC. Cholecystokinin / gastrin. In: Psychopharmacology: The fourth generation of progress, edited by Bloom FE, and Kupfer DJ. New York: Raven Press, Ltd., 1995.
- Bellissimo N, and Anderson GH. Cholecystokinin-A receptors are involved in food intake suppression in rats after intake of all fats and carbohydrates tested. J Nutr 133: 2319-2325, 2003. PMID: 12840200
- Berna MJ, and Jensen RT. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr Top Med Chem 7: 1211-1231, 2007. PMID: 17584143
- Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, and Liddle RA. An enteroendocrine cell – enteric glia connection revealed by 3D electron microscopy. PLoS One 9: e89881, 2014. PMID: 24587096
- Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, and Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125: 782-786, 2015. PMID: 25555217
- Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, Gribble FM, and Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology 156: 3961-3970, 2015. PMID: 26280129
- Castillo EJ, Delgado-Aros S, Camilleri M, Burton D, Stephens D, O'Connor-Semmes R, Walker A, Shachoy-Clark A, and Zinsmeister AR. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 287: G363-369, 2004. PMID: 15246968
- Chandra R, Samsa L, Vigna S, and Liddle R. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res 341: 289-297, 2010. PMID: 20582553
- Chandra R, and Liddle RA. Regulation of pancreatic secretion. Pancreapedia: Exocrine Pancreas Knowledge Base DOI: 10.3998/panc.2015.38: 2015.
- Chandra R, Hiniker A, Kuo Y-M, Nussbaum RL, and Liddle RA. α-synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2: e92295, 2017. PMID: 28614796
- Chandra R, Wang Y, Shahid RA, Vigna SR, Freedman NJ, and Liddle RA. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J Clin Invest 123: 3343-3352, 2013. PMID: 23863714
- Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, and Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol 304: G271-G282, 2013. PMID: 23203156
- Debas HT, Farooq O, and Grossman MI. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology 68: 1211-1217, 1975. PMID: 1126597
- Desai A, and Miller L. Cholecystokinin type 1 receptor Pancreapedia: Exocrine Pancreas Knowledge Base DOI: 10.3998/panc.2013.9: 2013.
- Dufresne M, Seva C, and Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 86: 805-847, 2006. PMID: 16816139
- Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, and Reeve JR, Jr. Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol 253: G477-482, 1987. PMID: 3661709
- Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, and Reeve JR. Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262: 214-217, 1987. PMID: 3793725
- Foltz M, Ansems P, Schwarz J, Tasker MC, Lourbakos A, and Gerhardt CC. Protein hydrolysates induce cck release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. J Agric Food Chem 56: 837-843, 2008. PMID: 18211011
- Friedman JM, Schneider BS, Barton DE, and Francke U. Level of expression and chromosome mapping of the mouse cholecystokinin gene: Implications for murine models of genetic obesity. Genomics 5: 463-469, 1989. PMID: 2575582
- Gabryelewicz A, Kulesza E, and Konturek SJ. Comparison of loxiglumide, a cholecystokinin receptor antagonist, and atropine on hormonal and meal-stimulated pancreatic secretion in man. Scand J Gastroenterol 25: 731-738, 1990. PMID: 2396088
- Gibbs J, Young RC, and Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 245: 323, 1973. PMID: 4586439
- Gibbs J, Young RC, and Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488-495, 1973. PMID: 4745816
- Green GM, and Lyman RL. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med 140: 6-12, 1972. PMID: 5033119
- Green GM. Feedback inhibition of cholecystokinin secretion by bile acids and pancreatic proteases. Ann N Y Acad Sci 713: 167-179, 1994. PMID: 8185158
- Haarstad H, Steffensrud S, Winnberg A, and Petersen H. The trophic effect on the pancreas of long-term continuous intravenous infusion of secretin and a cholecystokinin-like peptide in rats. Scand J Gastroenterol 21: 589-597, 1986. PMID: 2428095
- Haarstad H, and Petersen H. The effects of graded doses of a cholecystokinin-like peptide with and without secretin on pancreatic growth and synthesis of rna and polyamines in rats. Scand J Gastroenterol 24: 907-915, 1989. PMID: 2480634
- Harper AA, and Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol (Lond) 102: 115-125, 1943. PMID: 16991584
- Herzig KH, Schön I, Tatemoto K, Ohe Y, Li Y, Fölsch UR, and Owyang C. Diazepam binding inhibitor is a potent cholecystokinin-releasing peptide in the intestine. Proc Natl Acad Sci U S A 93: 7927-7932, 1996. PMID: 8755579
- Hildebrand P, Beglinger C, Gyr K, Jansen JB, Rovati LC, Zuercher M, Lamers CB, Setnikar I, and Stalder GA. Effects of a cholecystokinin receptor antagonist on intestinal phase of pancreatic and biliary responses in man. J Clin Invest 85: 640-646, 1990. PMID: 2312719
- Hoffmann P, Eberlein GA, Reeve JR, Jr., Bunte RH, Grandt D, Goebell H, and Eysselein VE. Comparison of clearance and metabolism of infused cholecystokinins 8 and 58 in dogs. Gastroenterology 105: 1732-1736, 1993. PMID: 8253350
- Ivy AC, and Oldberg E. A hormone mechanism for gall-bladder contraction and evacuation. Am J Physiol 86: 599-613, 1928.
- Jansen JB, Jebbink MC, Douglas BR, and Lamers CB. Effect of loxiglumide (CR-1505) on bombesin- and meal-stimulated plasma cholecystokinin in man. Eur J Clin Pharmacol 38: 367-370, 1990. PMID: 2344859
- Ji B, Bi Y, Simeone D, Mortensen RM, and Logsdon CD. Human pancreatic acinar cells do not respond to cholecystokinin. Pharmacol Toxicol 91: 327-332, 2002. PMID: 12688376
- Jo YH, Lee KY, Chang T-M, and Chey WY. Role of cholecystokinin in pancreatic bicarbonate secretion in dogs. Pancreas 6: 197-201, 1991. PMID: 1886888
- Johnsen AH. Phylogeny of the cholecystokinin/gastrin family. Front Neuroendocrinol 19: 73-99, 1998. PMID: 9578981
- Johnsen AH, Jønson L, Rourke IJ, and Rehfeld JF. Elasmobranchs express separate cholecystokinin and gastrin genes. Proc Natl Acad Sci U S A 94: 10221-10226, 1997. PMID: 9294191
- Jordan J, Greenway FL, Leiter LA, Li Z, Jacobson P, Murphy K, Hill J, Kler L, and Aftring RP. Stimulation of cholecystokinin-A receptors with GI181771X does not cause weight loss in overweight or obese patients. Clin Pharmacol Ther 83: 281-287, 2008. PMID: 17597711
- Kerstens PJ, Lamers CB, Jansen JB, de Jong AJ, Hessels M, and Hafkenscheid JC. Physiological plasma concentrations of cholecystokinin stimulate pancreatic enzyme secretion and gallbladder contraction in man. Life Sci 36: 565-569, 1985. PMID: 3968978
- Kissileff HR, Pi-Sunyer FX, Thornton J, and Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 34: 154-160, 1981. PMID: 6259918
- Konturek SJ, Kwiecien N, Obtulowicz W, Kopp B, Oleksy J, and Rovati L. Cholecystokinin in the inhibition of gastric secretion and gastric emptying in humans. Digestion 45: 1-8, 1990. PMID: 2340960
- Koop I, Schindler M, Bosshammer A, Scheibner J, Stange E, and Koop H. Physiological control of cholecystokinin release and pancreatic enzyme secretion by intraduodenal bile acids. Gut 39: 661-667, 1996. PMID: 9026479
- Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, and Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103: 383-391, 1999. PMID: 9927499
- Ku SK, Lee HS, and Lee JH. An immunohistochemical study of the gastrointestinal endocrine cells in the C57BL/6 mice. Anat Histol Embryol 32: 21-28, 2003. PMID: 12733269
- Lambrechts DAJE, Brandt-Wouters E, Verschuure P, Vles HSH, and Majoie MJM. A prospective study on changes in blood levels of cholecystokinin-8 and leptin in patients with refractory epilepsy treated with the ketogenic diet. Epilepsy Res 127: 87-92, 2016. PMID: 27568597
- Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, and Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112: 280-286, 1997. PMID: 8978369
- Larsson LI, and Rehfeld JF. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res 165: 201-218, 1979. PMID: 369662
- Li Y, and Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest 92: 418-424, 1993. PMID: 8100836
- Li Y, Hao Y, and Owyang C. Diazepam-binding inhibitor mediates feedback regulation of pancreatic secretion and postprandial release of cholecystokinin. J Clin Invest 105: 351-359, 2000. PMID: 10675361
- Liddle RA. On the measurement of cholecystokinin. Clin Chem 44: 903-904, 1998. PMID: 9590358
- Liddle RA, Morita ET, Conrad CK, and Williams JA. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest 77: 992-996, 1986. PMID: 3949984
- Liddle RA. Regulation of pancreatic secretion. In: Physiology of the Gastrointestinal Tract, edited by L. R. Johnson, F.K. Ghishan, J. D. Kaunitz, J. L. Merchant, H. M. Said, and J. D. Wood London: Academic Press, 2012, p. 1425-1460.
- Liddle RA, Gertz BJ, Kanayama S, Beccaria L, Coker LD, Turnbull TA, and Morita ET. Effects of a novel cholecystokinin (CCK) receptor antagonist, MK-329, on gallbladder contraction and gastric emptying in humans. Implications for the physiology of CCK. J Clin Invest 84: 1220-1225, 1989. PMID: 2794058
- Liddle RA. Regulation of pancreatic secretion. In: Physiology of the Gastrointestinal Tract, edited by L. R. Johnson. K. Barettt, F. K. Ghishan, J. L. Merchant, H. M. Said, J. D. Wood. London: Academic Press, 2006, p. 1397-1436.
- Liddle RA, Toskes PP, Horrow J, Ghali J, Dachman A, and Stong D. Lack of trophic pancreatic effects in humans with long-term administration of ximelagatran. Pancreas 32: 205-210, 2006. PMID: 16552342
- Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, and Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144-1152, 1985. PMID: 2580857
- Liddle RA, Goldfine ID, and Williams JA. Bioassay of plasma cholecystokinin in rats: Effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87: 542-549, 1984. PMID: 6204904
- Lieverse RJ, Jansen JB, Masclee AA, and Lamers CB. Satiety effects of a physiological dose of cholecystokinin in humans. Gut 36: 176-179, 1995. PMID: 7883212
- Lindén A, Carlquist M, Hansen S, and Uvnäs-Moberg K. Plasma concentrations of cholecystokinin, CCK-8, and CCK-33, 39 in rats, determined by a method based on enzyme digestion of gastrin before HPLC and RIA detection of CCK. Gut 30: 213-222, 1989. PMID: 2703143
- Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, and Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol 300: G538-G546, 2011. PMID: 21252045
- Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, and Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140: 903-912, 2011. PMID: 20955703
- Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, and Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol 294: R803-810, 2008. PMID: 18160529
- Matters GL, Cooper TK, McGovern CO, Gilius EL, Liao J, Barth BM, Kester M, and Smith JP. Cholecystokinin mediates progression and metastasis of pancreatic cancer associated with dietary fat. Dig Dis Sci 59: 1180-1191, 2014. PMID: 24817409
- McLaughlin J, Grazia Luca M, Jones MN, D'Amato M, Dockray GJ, and Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology 116: 46-53, 1999. PMID: 9869601
- Moran TH, and Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 286: G183-188, 2004. PMID: 14715515
- Nishimura S, Bilgüvar K, Ishigame K, Sestan N, Günel M, and Louvi A. Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development. PLoS One 10: e0124295, 2015. PMID: 25875176
- Ohlsson B, Rehfeld JF, and Axelson J. CCK stimulates growth of both the pancreas and the liver. Int J Surg Investig 1: 47-54, 1999. PMID: 11817337
- Olsson C, Aldman G, Larsson A, and Holmgren S. Cholecystokinin affects gastric emptying and stomach motility in the rainbow trout oncorhynchus mykiss. J Exp Biol 202: 161-170, 1999. PMID: 9851905
- Owyang C, May D, and Louie DS. Trypsin suppression of pancreatic enzyme secretion: Differential effect on cholecystokinin release and the enteropancreatic reflex. Gastroenterology 91: 637-643, 1986. PMID: 3732765
- Pauwels S, Najdovski T, Dimaline R, Lee CM, and Deschodt-Lanckman M. Degradation of human gastrin and CCK by endopeptides 24.11: Differential behaviour of the sulphated and unsulphated peptides. Biochim Biophys Acta 996: 82-88, 1989. PMID: 2736261
- Povoski SP, Zhou W, Longnecker DS, Jensen RT, Mantey SA, and Bell RH, Jr. Stimulation of in vivo pancreatic growth in the rat is mediated specifically by way of cholecystokinin-A receptors. Gastroenterology 107: 1135-1146, 1994. PMID: 7523219
- Pupovac J, and Anderson GH. Dietary peptides induce satiety via cholecystokinin-A and peripheral opioid receptors in rats. J Nutr 132: 2775-2780, 2002. PMID: 12221244
- Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol 7: 570-574, 2007. PMID: 17954038
- Raybould HE, Roberts ME, and Dockray GJ. Reflex decreases in intragastric pressure in response to cholecystokinin in rats. Am J Physiol 253: G165-170, 1987. PMID: 2887117
- Reeve JR, Liddle RA, Shively JE, Lee TD, Keire DA, Chew P, and Vigna SR. Sequence variation outside the "active"' region of dog and rabbit cholecystokinin-58 results in bioactivity differences. Pancreas 32: 306-313, 2006. PMID: 16628087
- Reeve JR, Jr., Green GM, Chew P, Eysselein VE, and Keire DA. Cck-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol 285: G255-265, 2003. PMID: 12686511
- Rehfeld JF. Cholecystokinin - from local gut hormone to ubiquitous messenger. Front Endocrinol (Lausanne) 8: 2017. PMID: 28450850
- Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem 44: 991-1001, 1998. PMID: 9590372
- Resin H, Stern DH, Sturdevant RAL, and Isenberg JI. Effect of the C-terminal octapeptide of cholecystokinin on lower esophageal sphincter pressure in man. Gastroenterology 64: 946-949, 1973. PMID: 4700420
- Schjoldager BT. Role of CCK in gallbladder function. Ann N Y Acad Sci 713: 207-218, 1994. PMID: 8185161
- Schmidt WE, Creutzfeldt W, Schleser A, Choudhury AR, Nustede R, Hocker M, Nitsche R, Sostmann H, Rovati LC, and Folsch UR. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: Effects of loxiglumide. Am J Physiol Gastrointest Liver Physiol 260: G197-G206, 1991. PMID: 1996640
- Singer MV, and Niebergall-Roth E. Secretion from acinar cells of the exocrine pancreas: Role of enteropancreatic reflexes and cholecystokinin. Cell Biol Int 33: 1-9, 2009. PMID: 18948215
- Solomon TE, Sayegh AI, and Reeve JR. CCK. In: Handbook of Biologically Active Peptides: Gastrointestinal Peptides, edited by Abba Kastin. Amsterdam: Elsevier Inc., 2013, p. 1197 - 1203.
- Spannagel AW, Green GM, Guan D, Liddle RA, Faull K, and Reeve JR, Jr. Purification and characterization of a luminal cholecystokinin-releasing factor from rat intestinal secretion. Proc Natl Acad Sci U S A 93: 4415-4420, 1996. PMID: 8633081
- Szalmay G, Varga G, Kajiyama F, Yang X-S, Lang TF, Case RM, and Steward MC. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol 535: 795-807, 2001. PMID: 11559776
- Takahashi Y, Fukushige S, Murotsu T, and Matsubara K. Structure of human cholecystokinin gene and its chromosomal location. Gene 50: 353-360, 1986. PMID: 3582983
- Takahashi Y, Kato K, Hayashizaki Y, Wakabayashi T, Ohtsuka E, Matsuki S, Ikehara M, and Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A 82: 1931-1935, 1985. PMID: 3856870
- Tanaka T, Katsuma S, Adachi T, Koshimizu T-A, Hirasawa A, and Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn-Schmiedeberg's Arch Pharmacol 377: 523-527, 2008. PMID: 17972064
- Tomita H, Miyasaka K, Matsumoto M, and Funakoshi A. Direct, concentration-dependent inhibition by taurocholate of pancreatic exocrine secretion and CCK release in conscious rats. Dig Dis Sci 39: 1544-1549, 1994. PMID: 8026268
- Turkelson CM, and Solomon TE. Molecular forms of cholecystokinin in rat intestine. Am J Physiol Gastrointest Liver Physiol 259: G364-G371, 1990. PMID: 2399981
- Wang BJ, and Cui ZJ. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am J Physiol Regul Integr Comp Physiol 292: R666-R678, 2007. PMID: 17053097
- Wang DQH, Schmitz F, Kopin AS, and Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest 114: 521-528, 2004. PMID: 15314689
- Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, and Liddle RA. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol 300: G528-537, 2011. PMID: 21183662
- Williams JA. Cholecystokinin: A hormone and a neurotransmitter. Biomedical Research 3: 107-121, 1982.
- Williams JA. Regulation of normal and adaptive pancreatic growth. Pancreapedia: Exocrine Pancreas Knowledge Base DOI: 10.3998/panc.2017.02: 2016.
- Wolkowitz OM, Gertz B, Weingartner H, Beccaria L, Thompson K, and Liddle RA. Hunger in humans induced by MK-329, a specific peripheral-type cholecystokinin receptor antagonist. Biol Psychiatry 28: 169-173, 1990. PMID: 2378921
- Wu XM, Liao YW, Ji KQ, Li GF, and Zang B. The trophic effect of cholecystokinin on the pancreas declines in rats on total parenteral nutrition. J Anim Physiol Anim Nutr (Berl) 96: 214-219, 2012. PMID: 21438927
- Yamagishi T, and Debas HT. Cholecystokinin inhibits gastric emptying by acting on both proximal stomach and pylorus. Am J Physiol 234: E375-378, 1978. PMID: 645853
- Zhou L, Yang H, Lin X, Okoro EU, and Guo Z. Cholecystokinin elevates mouse plasma lipids. PLoS One 7: e51011, 2012. PMID: 23300532