Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.4
| Attachment | Size |
|---|---|
| 625.05 KB |
Version 2.0 is the current version, it can be viewed here.
Protein symbols: PP2B, Cn
Genes: PPP3CA (CnAα); PPP3CB, (CnAβ); PPP3CC (CnAγ); PPP3R1 (CnB1); PPP3R2 (CnB2)
1. General information
Calcineurin (Cn) is a Ca2+/calmodulin (CaM)-dependent serine/threonine phosphatase first identified in extracts of mammalian brain (52, 70). Its name was further derived from its ability to bind Ca2+. Its importance has been documented in a number of physiologic and pathologic conditions including neuronal and muscle development, lymphocyte activation, cardiac hypertrophy, switching of skeletal muscle fiber type, and expression of ion channels. Cn (also known as PP2B) is part of a family of type 2 protein phosphatases that includes PP2A and PP2C. They are classified according to their dependence on certain divalent metal ions for phosphatase activity and Cn is uniquely dependent upon Ca2+. PP2A and PP1, but not Cn, are inhibited by the exogenously administered phosphatase inhibitors okadaic acid, microcystin, and calyculin, as well as the endogenous inhibitors inhibitor-1, DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa), and inhibitor-2, whereas Cn is specifically inhibited by the immunosuppressant drugs FK506 (tacrolimus) and cyclosporine A (CsA)(33).
There are several very good comprehensive reviews on Cn (4, 52), more recent brief updates (3, 28, 33), information on Cn inhibitors (31, 43), and disease or organ/tissue-specific reviews relating to Cn in the neurosciences (22), muscle (50), and islet cells (30).
Cn isoforms, structure, and function
Cn consists of two subunits, CnA and CnB, which form a heterodimer in order to conduct phosphatase activity. CnA (Fig. 1) contains the catalytic domain, which is homologous to other serine/threonine protein phosphatases (4). A dinuclear metal center composed of one iron and one zinc molecule lies next to a ß sandwich on the active site (Fig. 2). CnA also has 3 regulatory domains: a binding domain for its partner subunit CnB, a CaM binding domain, and an autoinhibitory domain. CnB, the regulatory subunit, contains 4 Ca2+-binding EF hand motifs that regulate through a conformational change the catalytic function of Cn. CnB binding to CnA may also facilitate proper folding of the active enzyme (15). CnB resembles CaM in that both bind to an extended a helix on their respective CnA domains. The primary sequence of the Cn subunit is highly conserved. CnA and CnB are tightly bound (kd = 10-13 M) even in the total absence of Ca2+. Two short a helices form the inhibitory domain and block the catalytic center under basal low Ca2+ conditions. The CaM binding domain is flexible. Binding of CaM along with Ca2+ binding to CnB induces displacement of the inhibitory domain, thus exposing the catalytic domain.

Fig. 1. Primary sequence and domain structure of CnA. The amino acid sequence represents rat CnAa. Note the regulatory domains that bind CnB and CaM as well as the autoinhibitory domain (AI). Modified from(52).


Fig. 2. Cn structure. (Top panel) Ribbon diagram of CnA (yellow) and CnB (blue). The former has several disordered regions (DRs), including a long stretch of 95 residues (red); the CaM binding a helix is contained within the DR. (Bottom panel) X-ray crystallography of CaM (blue) demonstrates binding to the a-helix of CnA (now colored yellow). Source: http://www.pondr.com/pondr-tut1.html
Cn isoforms and tissue distribution
CnA has 3 isoforms CnAa, CnAb, and CnAg. CnB has two isoforms CnB1 and CnB2. CnAb also has two splice variants which differ in their C-terminal domain (23). CnAa and CnAb appear to interact interchangeably with CnB1. Cn is highly enriched in brain; it constitutes 1% of total protein content and there are 20-30 fold greater amounts there than in other tissues. However, Cn is ubiquitously expressed and has differential isoform distribution. CnAg and CnB2 are primarily found in testis. There is greater abundance of CnAa over CnAb in brain and heart, but the reverse is true in spleen, thymus, and lymphocytes. CnAb is considered a stress-responsive isoform (8, 64).
The subcellular distribution of Cn is also distinct in certain cell types. Although Cn is localized to the cytoplasm in most systems, in spermatids, for example, it is localized to the nucleus; levels are most abundant during the initial stage of nuclear elongation with almost no signal present in the cytoplasm (45). In many systems, Cn co-translocates with NFAT (nuclear factor of activated T cells) to the nucleus upon activation by Ca2+ (58). In chicken forebrain, Cn is highly enriched in cytoplasmic, microsomal, and synaptosomal fractions (1). Cn is also co-localized with the cytoskeleton in cultured neurons(16) and the T-tubules of ventricular myocytes (54). The molecular mechanism of this targeting is not fully clear. However, calsarcin-1 and -2 tether Cn to a-actinin and may couple Cn activity with muscle contraction (17).
Regulation of Cn
Several factors regulate Cn. The most potent activators are Ca2+ and CaM. As mentioned earlier, even at low cytosolic Ca2+ concentrations, CnA is tightly bound to CnB (38). However, a sustained rise in Ca2+ causes the dual recruitment of CaM to CnA and the binding of Ca2+ to CnB. This results in a conformational change in CnA that forces its autoinhibitory domain to dissociate from its catalytic groove, thereby permitting Cn activity. Of the two initiating components, it is thought that CaM is the critical activator. Cn is also reversibly inactivated by oxidation of its Fe2+ molecule (71). In fact, there is some thought that a Ca2+/CaM-induced conformational change in Cn exposes the Fe2+ to oxidation, thus providing negative feedback for Cn activation.
A number of endogenous Cn inhibitors have been identified. They include AKAP79 (A-kinase anchoring protein of 79 kDa), which as its name implies also anchors PKA with Cn (13). Another protein with this dual anchoring and inhibitory action on Cn in cardiac and skeletal muscle is calsarcin (17). A family of proteins called modulatory Cn interacting proteins (MCIPs) serves as feedback inhibitors of Cn (51). In humans, its gene was initially identified as DSCR1 (Downs’s syndrome critical region 1) (61). A recent consensus was reached to call these proteins regulators of Cn (RCANs) (11). They are upregulated by Cn-mediated activation of the transcription factor NFAT. They can inhibit Cn and interestingly also directly inhibit NFAT through binding a highly conserved ISPPxSPP motif found on both proteins. Other inhibitors include Cn homologous protein (CHP) and Cain/Cabin1 (35). The latter is a 240 kDa nuclear protein that inhibits Cn in a Ca2+- and PKC-dependent manner likely by binding to the same site as FK506-FKBP. Other factors contributing to Cn activation include polyunsaturated fatty acids (32).
Cn targets
Cn has a number of phospho-protein targets identified. The best known is NFAT, which resides in the cytoplasm during basal conditions, but translocates to the nucleus upon dephosphorylation by Cn (41). Other targets of Cn include CRHSP-24 (Ca2+-regulated heat stable protein of 24kDa) (21,55), DARPP-32 (48) and inhibitor-1 (46) (inhibitor of protein phosphatase 1), the regulatory RII subunit of cAMP dependent protein kinase (6), Bcl2 family member BAD (69), a CaM binding protein neuromodulin (40), NO synthase (12), adenylate cyclase (2), HSP70 (36), endocytic component GTPase dynamin1 (39), transcription factors MEF2 (76) and Elk-1 (62), Gap43 (7), and microtubule-associated proteins Map-2, tau, and tubulin (18). Cn may also provide a feedback mechanism for maintaining cellular Ca2+ homeostasis by regulating the expression of several ion channels channels (9).
Cn in physiology and disease
As mentioned earlier, Cn is highly enriched in neurons, but it is ubiquitously expressed in all tissues and cells. In the brain, it functions to activate a series of phosphatases by dephosphorylating the endogenous inhibitors of PP-1: inhibitor-1 and DARPP-32. Cn thus intricately regulates synaptic plasticity and long term memory (42, 77). Cn also regulates synaptic vesicle endocytosis by dephosphorylating the dephosphins (10). In T cells, sustained cytosolic Ca2+ release leads to Cn/NFAT activation and the induction of T cell activating genes, notably interleukin-2 (41). The reason why the Cn inhibitors FK506 and CsA are such effective chronic immunosuppressive drugs is this blockade of T cell Cn. In heart, the Cn/NFAT pathway may protect against dilated cardiomyopathy (26). However, it plays a pathologic role in cardiac hypertrophy (44, 63, 74), through either activation of NFAT3 (along with GATA4), co-activation of NFAT and MEF2, or PKC activation. In skeletal muscle, Cn regulates, again through NFAT3 and MEF2, switching of muscle fiber subtype (50, 76). In islet cells Cn/NFAT regulates beta cell growth (27, 30) and survival (5, 60).
2. Cn in the exocrine pancreas
Investigations in the exocrine pancreas relating to Cn have focused on the acinar cell. Cn was reported to inhibit acinar cell exocytosis of pancreatic enzymes (72). Initial work in both pancreatic lobules as well as dispersed acini demonstrated that CsA and FK506 each reduced caerulein and carbachol-stimulated amylase secretion (14, 72). However, later studies could reproduce only a modest reduction using FK506 (20, 29). Further work showed that Cn is required for translational control of acinar cell protein synthesis. In isolated acinar cells stimulated with either CCK, bombesin, or carbachol, FK506 reduced methionine incorporation into protein. FK506 modulated factors in the translation machinery: it reduced the phosphorylation of mRNA cap binding protein eukaryotic initiation factor (eIF) 4E binding protein, reduced the formation of the eIF4F complex, and increased the phosphorylation of eukaryotic elongation factor 2 (53). In a series of elegant studies using an experimental model of adaptive growth in which mice were fed the soybean trypsin inhibitor camostat in order to stimulate endogenous CCK release, pancreatic growth was shown to be dependent upon Cn (25, 65). This was initially demonstrated using CsA and FK506 (14, 66). Cn pathways could explain several important aspects of pancreatic growth, such as c-Jun NH2-terminal kinase activation (65). In a subsequent study, overexpression of the endogenous Cn inhibitor Rcan1 selectively within acinar cells also led to reduced adaptive growth of the pancreas (24). Because Rcan1 is a transcriptional target of NFAT, validated in the acinar cell by chromatin immunoprecipitation, it was suggested that Cn modulates growth through NFAT activation (24). Indeed, using an NFAT-luciferase reporter, CCK activated NFAT signaling (25). Cn was also shown to mediate experimental pancreatitis (29, 56). FK506 administration in vivo attenuated the severity of pancreatitis induced by hyperstimulation with the CCK analog caerulein. Further, the Cn inhibitor FK506 and Cn inhibitory peptide (CiP) reduced pathologic intra-acinar protease activation, an early marker of pancreatic injury. Although NFAT pathways have primarily been investigated, other substrates that mediate non-transcriptional events may play a role in the exocrine pancreas. Notably, the Cn substrate CRHSP-24 was first identified in the exocrine pancreas (21, 37).
3. Tools for the study of Cn
a. Molecular constructs
Several Cn clones and adenoviral constructs have been created. A major tool employed to identify a role for Cn in a cellular process has been the overexpression of a CaM-independent derivative of CnAa residues (1-392), in effect producing a constitutively active Cn (?CnA)(49). A host of plasmids are available for purchase at Addgene (www.addgene.org). Adenoviral vectors are available from Seven Hills Bioreagents. siRNA for Cn is available through Ambion.
b. Antibodies
In our experience, commercially available Cn antibodies are not particularly specific for their labeled Cn isoforms, particularly the CnAß antibody. However, they are available from Santa Cruz, Upstate Biotech, BD Transduction Labs, and Chemicon. Most of them, however, can be used for immunofluorescence in addition to western blotting.
c. Transgenic mice
CnAb knockout mice were made by Dr. Jeff Molkentin (8). They live to adulthood, breed well, and have no gross phenotypic defects. CnAa knockout mice were made by Dr. Jon Seidman (78). CnB1 knockout mice do not live beyond the embryonic period due to fatal defects in vascular patterning. However, floxed CnB1 mice were made by Dr. Gerald Crabtree (47). Floxed CnAb mice are also available. To our knowledge, CnAg-/- or CnB2-/- are not available. An NFAT luciferase reporter mice has been used to monitor Cn activation (73).
d. Cn Activity
Cn activity is primarily measured in vitro using a 19 residue synthetic peptide corresponding to residues 81-99 of the RII subunit of cAMP-dependent protein kinase(6). As an alternative Biomol Labs carries a colorimetric assay kit. In vivo measurements can be performed by monitoring NFAT nuclear translocation. In pancreas the dephosphorylation of CRSP-24 has also been used. A transgenic mouse that expresses an NFAT luciferase reporter was made by Dr. Jeff Molkentin(73).
e. Pharmacologic inhibitors
The two prototypic Cn inhibitors, FK506 and CsA, form a complex with FK506 binding protein (FKBP12) and cyclophilin, respectively (43). The complex then binds Cn and blocks access of substrates to its catalytic site. The junction between CnB and CnA has been identified by crystallography to be the binding site for FK506 (Fig. 3). The two inhibitors are widely used in clinical practice as immunosuppressants after organ transplantation or for the treatment of autoimmune disorders because they diminish Cn-dependent T cell activation. Several novel variations of these inhibitors are in testing (59). FK506 is more specific than CsA. The latter can bind cyclophilin D and thereby inhibit the mitochondrial permeability transition pore (75). It should also be noted that both FK506 and CsA have worrisome side effects with prolonged, chronic use, such as hypertension and neurotoxicity(68).
CiP is a short peptide that mimics the auto-inhibitory domain of Cn. A cell permeant form of CiP, made by covalently attaching an arginine tail to the peptide is available through Calbiochem (67). As a negative control, however, it will be important to synthesize a scrambled peptide of equal length that also has an arginine tail.
Phosphatase inhibitors that do not inhibit Cn can be used as negative controls to determine the selectivity of an effect for Cn. In particular, the serine/threonine phosphatases PP1 and PP2A can be inhibited by okadaic acid (IC50 20 nM and 0.2 nM, respectively), calyculin-A (IC50 1 nM for both), and microcystin-LR (IC50 0.1 nM for both) (57). The former two inhibitors have been used in pancreatic acinar cells (55), and none inhibit Cn (PP2B) at the noted concentrations.
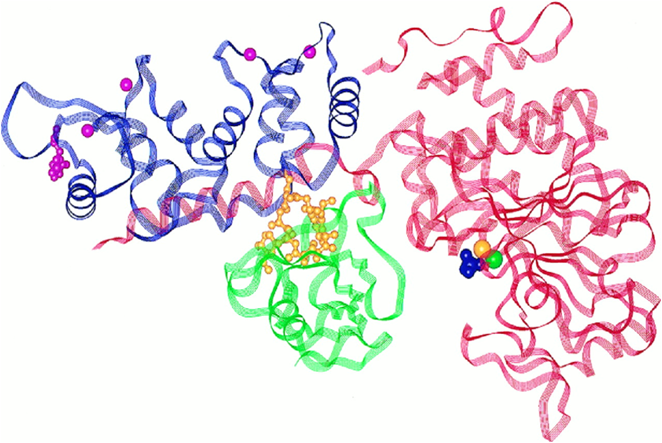
Fig. 3. Ribbon diagram of truncated Cn complexed with FK506-FKBP12. CnA is shown in red and CnB in purple, with myristic acid covalently linked to the N-terminal glycine shown in pink. Iron and zinc are contained within the active site of CnA (yellow and green spheres, respectively), and the bound phosphate is shown in purple. Four molecules of Ca2+ on their respective CnB binding sites are shown as pink spheres. The FK506 (yellow)-FKBP12 (green) complex blocks entry of Cn substrates to the active site on CnA. (Protein Data Bank code 1TCO(19)). Modified from(34).
4. References
- Anthony FA, Winkler MA, Edwards HH, and Cheung WY. Quantitative subcellular localization of calmodulin-dependent phosphatase in chick forebrain. J Neurosci 8: 1245-1253, 1988. PubMed PMID: 2833579.
- Antoni FA, Smith SM, Simpson J, Rosie R, Fink G, and Paterson JM. Calcium control of adenylylcyclase: the calcineurin connection. Advances in second messenger and phosphoprotein research 32:153-172, 1998. PubMed PMID: 9421590.
- Aramburu J, Heitman J, and Crabtree GR. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep 5: 343-348, 2004. PubMed PMID: 15060569.
- Aramburu J, Rao A, and Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul 36:237-295, 2000. PubMed PMID: 10842755.
- Bernal-Mizrachi E, Cras-Meneur C, Ye BR, Johnson JD, and Permutt MA. Transgenic overexpression of active calcineurin in beta-cells results in decreased beta-cell mass and hyperglycemia. PLoS One 5: e11969, 2010. PubMed PMID: 20689817.
- Blumenthal DK, Takio K, Hansen RS, and Krebs EG. Dephosphorylation of cAMP-dependent protein kinase regulatory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J Biol Chem 261: 8140-8145, 1986. PubMed PMID: 3013843.
- Bolsover SR. Calcium signalling in growth cone migration. Cell Calcium 37: 395-402, 2005. PubMed PMID: 15820386.
- Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, and Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A 99:4586-4591, 2002. PubMed PMID: 11904392.
- Bultynck G, Vermassen E, Szlufcik K, De Smet P, Fissore RA, Callewaert G, Missiaen L, DeSmedt H, and Parys JB. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochemical and Biophysical Research Communications 311: 1181-1193, 2003. PubMed PMID: 14623304.
- Cousin MA, Tan TC, and Robinson PJ. Protein phosphorylation is required for endocytosis in nerve terminals: potential role for the dephosphins dynamin I and synaptojanin, but not AP180 or amphiphysin. J Neurochem 76: 105-116, 2001. PubMed PMID: 11145983.
- Davies KJ, Ermak G, Rothermel BA, Pritchard M, Heitman J, Ahnn J, Henrique-Silva F, Crawford D, Canaider S, Strippoli P, Carinci P, Min KT, Fox DS, Cunningham KW, Bassel-Duby R, Olson EN, Zhang Z, Williams RS, Gerber HP, Perez-Riba M, Seo H, Cao X, Klee CB, Redondo JM, Maltais LJ, Bruford EA, Povey S, Molkentin JD, McKeon FD, Duh EJ, Crabtree GR, Cyert MS, de la Luna S, and Estivill X. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J 21: 3023-3028, 2007. PubMed PMID: 17595344.
- Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Uhl GR, and Snyder SH. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A 90: 9808-9812, 1993. PubMed PMID: 7694293.
- Dell'Acqua ML, Dodge KL, Tavalin SJ, and Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J Biol Chem 277: 48796-48802, 2002. PubMed PMID: 12354762.
- Doi R, Inoue K, Chowdhury P, Kaji H, and Rayford PL. Structural and functional changes of exocrine pancreas induced by FK506 in rats. Gastroenterology 104: 1153-1164, 1993. PubMed PMID: 7681795.
- Feng B, and Stemmer PM. Interactions of calcineurin A, calcineurin B, and Ca2+. Biochemistry 38: 12481-12489, 1999. PubMed PMID: 10493818.
- Ferreira A, Kincaid R, and Kosik KS. Calcineurin is associated with the cytoskeleton of cultured neurons and has a role in the acquisition of polarity. Mol Biol Cell 4: 1225-1238, 1993. PubMed PMID: 8167406.
- Frey N, Richardson JA, and Olson EN. Calsarcins, a novel family of sarcomeric calcineurinbinding proteins. Proc Natl Acad Sci U S A 97: 14632-14637, 2000. PubMed PMID: 11114196.
- Goto S, Yamamoto H, Fukunaga K, Iwasa T, Matsukado Y, and Miyamoto E. Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J Neurochem 45: 276-283,1985. PubMed PMID: 2987415.
- Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, Fleming MA, Caron PR, Hsiao K, and Navia MA. X-ray structure of calcineurin inhibited by the immunophilinimmunosuppressant FKBP12-FK506 complex. Cell 82: 507-522, 1995. PubMed PMID: 7543369.
- Groblewski GE, Wagner AC, and Williams JA. Cyclosporin A inhibits Ca2+/calmodulindependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem 269: 15111-15117,1994. PubMed PMID: 7515049.
- Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, and Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem 273:22738-22744, 1998. PubMed PMID: 9712905.
- Groth RD, Dunbar RL, and Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun 311: 1159-1171, 2003. PubMed PMID: 14623302.
- Guerini D, and Klee CB. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A 86: 9183-9187, 1989. PubMed PMID: 2556704.
- Gurda GT, Crozier SJ, Ji B, Ernst SA, Logsdon CD, Rothermel BA, and Williams JA. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology 139: 609-619, 619 e601-606, 2010. PubMed PMID: 20438729.
- Gurda GT, Guo L, Lee SH, Molkentin JD, and Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Molecular Biology of the Cell 19: 198-206, 2008. PubMed PMID: 17978097.
- Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, and Molkentin JD. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol 48: 1080-1087, 2010. PubMed PMID: 19854199.
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, and Kim SK. Calcineurin/NFAT signalling regulates pancreatic [beta]-cell growth and function. Nature 443: 345-349, 2006. PubMed PMID: 16988714.
- Hogan PG, and Li H. Calcineurin. Current Biology 15: R442-R443, 2005. PubMed PMID: 15964258.
- Husain SZ, Grant WM, Gorelick FS, Nathanson MH, and Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 292: G1594-1599, 2007. PubMed PMID: 17332472.
- Heit JJ. Calcineurin/NFAT signaling in the beta-cell: From diabetes to new therapeutics. BioEssays 29: 1011-1021, 2007. PubMed PMID: 17876792.
- Kapturczak MH, Meier-Kriesche HU, and Kaplan B. Pharmacology of calcineurin antagonists. Transplantation Proceedings 36: 25S-32S, 2004. PubMed PMID: 15041303.
- Kessen U, Schaloske R, Aichem A, and Mutzel R. Ca2+/calmodulin-independent activation of calcineurin from Dictyostelium by unsaturated long chain fatty acids. J Biol Chem 274: 37821-37826, 1999. PubMed PMID: 10608845.
- Klee C, and Yang S. Calcineurin. In: Handbook of Cell Signaling. Elsevier, 2010, p. 705-710.
- Klee CB, Ren H, and Wang X. Regulation of the Calmodulin-stimulated Protein Phosphatase, Calcineurin. J Biol Chem 273: 13367-13370, 1998. PubMed PMID: 9593662.
- Lai MM, Burnett PE, Wolosker H, Blackshaw S, and Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem 273: 18325-18331, 1998. PubMed PMID: 9660798.
- Lakshmikuttyamma A, Selvakumar P, and Sharma RK. Interaction between heat shock protein 70 kDa and calcineurin in cardiovascular systems (Review). Int J Mol Med 17: 419-423, 2006. PubMed PMID: 16465387.
- Lee S, Wishart MJ, and Williams JA. Identification of calcineurin regulated phosphorylation sites on CRHSP-24. Biochem Biophys Res Commun 385: 413-417, 2009. PubMed PMID: 19477163.
- Li J, Jia Z, Zhou W, and Wei Q. Calcineurin regulatory subunit B is a unique calcium sensor that regulates calcineurin in both calcium-dependent and calcium-independent manner. Proteins 77: 612-623, 2009. PubMed PMID: 19536897.
- Liu JP, Sim AT, and Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science 265: 970-973, 1994. PubMed PMID: 8052858.
- Liu YC, and Storm DR. Dephosphorylation of neuromodulin by calcineurin. J Biol Chem 264:12800-12804, 1989. PubMed PMID: 2546935.
- Macian F. NFAT proteins: Key regulators of t-cell development and function. Nature Reviews Immunology 5: 472-484, 2005. PubMed PMID: 15928679.
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, and Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104: 675-686, 2001. PubMed PMID: 11257222.
- Martínez-Martínez S, and Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Current medicinal chemistry 11: 997-1007, 2004. PubMed PMID: 15078162.
- Molkentin JD, Lu J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, and Olson EN. A Calcineurin-Dependent Transcriptional Pathway for Cardiac Hypertrophy. Cell 93: 215-228, 1998. PubMed PMID: 9568714.
- Moriya M, Fujinaga K, Yazawa M, and Katagiri C. Immunohistochemical localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the mouse testis: its unique accumulation in spermatid nuclei. Cell and tissue research 281: 273-281, 1995. PubMed PMID: 7648621.
- Mulkey RM, Endo S, Shenolikar S, and Malenka RC. Involvement of a calcineurin/ inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486-488, 1994. PubMed PMID: 7515479.
- Neilson JR, Winslow MM, Hur EM, and Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 20: 255-266, 2004. PubMed PMID: 15030770.
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, and Greengard P. Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. Journal of Neurochemistry 81: 832-841, 2002. PubMed PMID: 12065642.
- O'Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, and O'Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 357: 692-694, 1992. PubMed PMID: 1377361.
- Olson EN, and Williams RS. Calcineurin signaling and muscle remodeling. Cell 101: 689-692,2000. PubMed PMID: 10892739.
- Rothermel BA, Vega RB, and Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med 13: 15-21, 2003. PubMed PMID: 12554096.
- Rusnak F, and Mertz P. Calcineurin: Form and Function. Physiol Rev 80: 1483-1521, 2000. PubMed PMID: 11015619.
- Sans MD, and Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am J Physiol Cell Physiol 287: C310-319, 2004. PubMed PMID 15044154.
- Santana LF, Chase EG, Votaw VS, Nelson MT, and Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol (Lond) 544: 57-69, 2002. PubMed PMID: 12356880.
- Schafer C, Steffen H, Krzykowski KJ, Goke B, and Groblewski GE. CRHSP-24 phosphorylation is regulated by multiple signaling pathways in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 285: G726-734, 2003. PubMed PMID: 12801884.
- Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Shah AU, Liu J, Mistry PK, Jain D, and Husain SZ. Protease Activation during in vivo Pancreatitis is Dependent upon Calcineurin Activation. Am J Physiol Gastrointest Liver Physiol 2009. PubMed PMID 20501444.
- Sheppeck JE, Gauss C-M, and Chamberlin AR. Inhibition of the ser-thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorganic & Medicinal Chemistry 5: 1739-1750, 1997. PubMed PMID: 9354230.
- Shibasaki F, Price ER, Milan D, and McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature 382: 370-373, 1996. PubMed PMID: 8684469.
- Sieber M, and Baumgrass R. Novel inhibitors of the calcineurin/NFATc hub - alternatives to CsA and FK506? Cell Commun Signal 7: 25, 2009. PubMed PMID: 19860902.
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De Leon DD, Naji A, Kushner JA, and Stoffers DA. Calcineurin signaling regulates human islet {beta}-cell survival. J Biol Chem 285: 40050-40059, 2010. PubMed PMID: 20943662.
- Strippoli P, Lenzi L, Petrini M, Carinci P, and Zannotti M. A new gene family including DSCR1 (Down Syndrome Candidate Region 1) and ZAKI-4: characterization from yeast to human and identification of DSCR1-like 2, a novel human member (DSCR1L2). Genomics 64: 252-263, 2000. PubMed PMID: 10756093.
- Sugimoto T, Stewart S, and Guan KL. The calcium/calmodulin-dependent protein phosphatase calcineurin is the major Elk-1 phosphatase. J Biol Chem 272: 29415-29418, 1997. PubMed PMID: 9367995.
- Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, and Molkentin JD. Prevention of Cardiac Hypertrophy in Mice by Calcineurin Inhibition. Science 281: 1690-1693, 1998. PubMed PMID: 9733519.
- Taigen T, De Windt LJ, Lim HW, and Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. PNAS 97: 1196-1201, 2000. PubMed PMID: 10655507.
- Tashiro M, Dabrowski A, Guo L, Sans MD, and Williams JA. Calcineurin-dependent and Calcineurin-independent signal transduction pathways activated as part of pancreatic growth. Pancreas 32: 314-320, 2006. PubMed PMID 16628088.
- Tashiro M, Samuelson LC, Liddle RA, and Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784-790, 2004. PubMed PMID: 14684381.
- Terada H, Matsushita M, Lu Y-F, Shirai T, Li S-T, Tomizawa K, Moriwaki A, Nishio S, Date I, Ohmoto T, and Matsui H. Inhibition of excitatory neuronal cell death by cell-permeable calcineurin autoinhibitory peptide. Journal of Neurochemistry 87: 1145-1151, 2003. PubMed PMID: 14622094.
- van Rossum HH, de Fijter JW, and van Pelt J. Pharmacodynamic monitoring of calcineurin inhibition therapy: principles, performance, and perspectives. Ther Drug Monit 32: 3-10, 2010. PubMed PMID: 20009796.
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, and Reed JC. Ca2+induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339-343, 1999. PubMed PMID: 10195903.
- Wang JH, and Desai R. A brain protein and its effect on the Ca2+-and protein modulator-activated cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun 72: 926-932, 1976. PubMed PMID: 186066.
- Wang X, Culotta VC, and Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature 383: 434-437, 1996. PubMed PMID: 8837775.
- Waschulewski IH, Hall DV, Kern HF, and Edwardson JM. Effects of the immunosuppressants cyclosporin A and FK 506 on exocytosis in the rat exocrine pancreas in vitro. Br J Pharmacol 108: 892-900, 1993. PubMed PMID: 7683567.
- Wilkins BJ, Dai Y-S, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, and Molkentin JD. Calcineurin/NFAT Coupling Participates in Pathological, but not Physiological, Cardiac Hypertrophy. Circ Res 94: 110-118, 2004. PubMed PMID: 14656927.
- Wilkins BJ, and Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and Biophysical Research Communications 322: 1178-1191, 2004. PubMed PMID: 15336966.
- Woodfield K, Ruck A, Brdiczka D, and Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 336 ( Pt 2): 287-290, 1998. PubMed PMID: 9820802.
- Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, and Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. Embo J 20: 6414-6423, 2001. PubMed PMID: 11707412.
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, and Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107: 617-629, 2001. PubMed PMID: 11733061.
- Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O'Neill EA, Seidman CE, Abbas AK, and Seidman JG. T cell responses in calcineurin A alpha-deficient mice. J Exp Med 183:413-420, 1996. PubMed PMID: 8627154.


