Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2022.03
| Attachment | Size |
|---|---|
| 301.96 KB |
Gene Symbol: ADM
1. General Information
Adrenomedullin (ADM) is a 52 amino acid peptide isolated by Kitamura et al from a human pheochromocytoma in 1993 that has a strong hypotensive action in anesthetized rats (16, 17). It is structurally homologous to calcitonin (CT), calcitonin-gene related peptide (CGRP), and amylin which are considered to form a family, the CT/CGRP family (8). It is now known to be expressed in various tissues of the human body particularly the adrenal medulla, the heart and the lungs but also in other tissues including the pancreatic islets (22). However, with the discovery that it is most highly expressed in endothelial and vascular smooth muscle cells, the peptide is regarded as a product of the vascular endothelium.
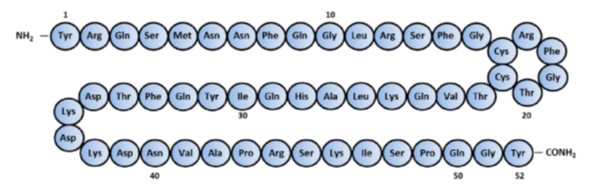
The peptide has an amidated carboxy terminal which is characteristic of bioactive peptides and two cysteines forming a disulfide bond to produce a ring structure of six amino acids (Figure 1). The gene encoding ADM is located on chromosome 11 in humans and chromosome 7 in mice (12) and produces a pre-proadrenomedullin of 185 amino acids. After the amino terminal signal peptide is removed, the remainder is processed to yield multiple peptides one of which is ADM-Gly (95-147). The C-terminal glycine is converted to a C-terminal amidation yielding mature ADM. Another peptide with biological activity in the precursor is proadrenomedullin N-terminal 20 peptide (PAMP) consisting of the first 20 amino acids of Pro-ADM which also has direct vasodilator action. More detail on the processing can be found in the review by Schӧnauer et al. (28).
Figure 1. Amino-acid sequence of human Adrenomedulin. (Reproduced from Ashizuka et al. (4)).
A second form of ADM termed ADM2 or intermedin was also identified in 2004 (11). Its biological effects resemble those of ADM but it is sometimes more potent. However, gene deletion shows that it is not essential.
ADM is present in human plasma with normal concentrations between 1 and 10 pM; the glycine extended form is also present at similar concentrations (10). The plasma half-life of ADM has been estimated as 22 minutes. It is not clear which tissues contribute the most to plasma ADM but it is known there is no increase with catecholamine secretion, so it is not the adrenal medulla. The short plasma half-life limits its therapeutic use although polyethylene-glycol (PEG) conjugates have been used to increase the half-life (8).
Early studies showed that ADM could activate some receptors for the related peptide, CGRP (10). However, studies with 125I-ADM showed specific receptors on rat vascular smooth muscle cells (7, 13) that bound ADM with 10-20 times higher affinity than CGRP. Results for a number of tissues and cell lines are compiled in Hinson et al. (10). In these studies, the human adrenomedullin fragment, ADM 22-52 acted as a specific antagonist.
Molecular cloning studies identified the calcitonin receptor-like receptor (CRLR) as a Class B G protein coupled receptor in 1993. CRLR were homologous to the calcitonin receptor and identified in the lung, heart and kidney. However, their function requires coexpression of another protein, the receptor activity modifying protein (RAMP), a single pass membrane protein that in part acts as a chaperone for insertion of the receptor protein and affects the ligand specificity (24, 32). There are three forms of RAMP and the AM1 receptor specific for ADM is made up of CRLR combined with RAMP2. The AM2 receptor contains CRLR and RAMP3 and has affinity for both ADM and CGRP. The CGRP receptor which does not bind ADM is made up of CRLR plus RAMP1. In the more recent literature CRLR is referred to as CLR (9). AM receptors almost always activate adenyl cyclase and increase cyclic AMP but in some cell types they also activate phospholipase C and mobilize intracellular Ca2+ (10, 29). It remains unclear whether a separate ADM2 receptor exists.
ADM has pleotropic actions in multiple organs and tissues (14). Most studies of ADM action have involved the heart and vasculature where it dilates blood vessels. A unique biological effect is to stimulate angiogenesis. Gene deletion studies have involved the heart and vasculature and have shown that ADM, CLR and RAMP2 are necessary for development of the heart in mice (5, 6). Mice without ADM die between day 13.5 and 14.5 with hydops fetalis (generalized edema). In rat ventricular myocytes ADM has both contraction and relaxation effects mediated by Gs and Gi respectively (23). ADM is produced in the heart and acts locally to affect contractility, hypertrophy and fibrosis. Its plasma level is increased in patients with heart failure and other cardiovascular disease. ADM is a protective factor in the cardiovascular system and is currently being explored as a therapeutic agent for heart failure and myocardial infarction (31).
ADM is also known to have multiple effects in the GI tract including anti-inflammatory actions, restoration of barrier function and effects on the GI microbiome (4, 19). ADM administered in the lumen was first shown to reduce inflammatory ulcers produced by acetic acid (3) and colitis induced by dextran sulphate in mice (2). Clinical trials using ADM in ulcerative colitis and Crohn’s disease are underway in Japan (15).
2. Role of Adrenomedullin in the Pancreas
Evidence exists for ADM playing a role in the pancreas primarily in the islets but also on acinar cells. In the rat embryo, ADM is localized to all islet endocrine cells but in the adult, it is primarily in the PP or F cells (20, 34). In most mammals and birds, ADM is located in the islets but in some lower vertebrates it is present in endocrine cells scattered among the acini (18). ADM is present in secretory granules in these cells. By contrast, ADM receptors are present on islet cells especially β cells (21). There is evidence that ADM inhibits insulin secretion, that antibody to ADM potentiates insulin secretion, and in vivo that ADM will increase plasma glucose (22). This study appears well done but needs confirmation especially since another study reported that ADM stimulated insulin secretion (25).
The other pancreatic cell type reported to be regulated by ADM is the acinar cell. Tsuchida et al. (30) showed that 125I-ADM specifically bound to isolated acini where it was displaced by ADM but not CGRP (30). ADM did not affect CCK binding to its receptor or the increase in intracellular Ca2+ induced by CCK but inhibited the release of amylase. The authors concluded that ADM inhibited acinar cells at a late step in secretion. This inhibition by ADM appeared to be mediated by a pertusis toxin sensitive G protein. While this study appears well done confirmation by another laboratory is needed. There is also one study that ADM can reduce cerulein induced experimental pancreatitis in rats, which may be related to its anti-inflammatory actions (26).
There is also evidence that ADM may be involved in diabetes. In type 2 diabetes, plasma ADM is increased but it is not clear if this is a cause or effect (33). ADM may also play a role in or be a marker of diabetes associated with early pancreatic cancer. ADM is upregulated in pancreatic cancer and in pancreatic cancer cell lines (1). Patients with pancreatic cancer frequently have diabetes 2-3 years before being diagnosed with pancreatic cancer and older patients with diabetes have an increased chance of being diagnosed with pancreatic cancer (27). These authors suggest that ADM is a potential mediator of islet disfunction seen in early pancreatic cancer.
3. Tools for Studying Adrenomedullin
a. Synthetic adrenomedullin peptide is available from most peptide companies such as My Biosource and Abcepta. The peptide antagonist, Adrenomedulin 22-52 is available from Sigma chemicals and Abcam.
b. Rabbit antibody to ADM suitable for Western blotting is available from Abcam (ab 190819). A monoclonal Ab suitable for IHC is available from In Vitrogen.
4. References
- Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ, Rumie Vittar NB, de Andrade M, Mukhopadhyay D, Petersen GM, Fernandez-Zapico ME, Logsdon CD, and Chari ST. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 143: 1510-1517 e1511, 2012. PMID: 22960655.
- Ashizuka S, Inagaki-Ohara K, Kuwasako K, Kato J, Inatsu H, and Kitamura K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol 53: 573-581, 2009. PMID: 19780971.
- Ashizuka S, Ishikawa N, Kato J, Yamaga J, Inatsu H, Eto T, and Kitamura K. Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides 26: 2610-2615, 2005. PMID: 15978699.
- Ashizuka S, Kita T, Inatsu H, and Kitamura K. Adrenomedullin: A Novel Therapeutic for the Treatment of Inflammatory Bowel Disease. Biomedicines 9: 2021. PMID: 34440272.
- Caron KM, and Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A 98: 615-619, 2001. PMID: 11149956.
- Dackor R, Fritz-Six K, Smithies O, and Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem 282: 18094-18099, 2007. PMID: 17470425.
- Eguchi S, Hirata Y, Kano H, Sato K, Watanabe Y, Watanabe TX, Nakajima K, Sakakibara S, and Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett 340: 226-230, 1994. PMID: 8131850.
- Fischer JP, Els-Heindl S, and Beck-Sickinger AG. Adrenomedullin - Current perspective on a peptide hormone with significant therapeutic potential. Peptides 131: 170347, 2020. PMID: 32569606.
- Hay DL, Garelja ML, Poyner DR, and Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol 175: 3-17, 2018. PMID: 29059473.
- Hinson JP, Kapas S, and Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21: 138-167, 2000. PMID: 10782362.
- Hong Y, Hay DL, Quirion R, and Poyner DR. The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol 166: 110-120, 2012. PMID: 21658025.
- Ishimitsu T, Kojima M, Kangawa K, Hino J, Matsuoka H, Kitamura K, Eto T, and Matsuo H. Genomic structure of human adrenomedullin gene. Biochem Biophys Res Commun 203: 631-639, 1994. PMID: 8074714.
- Ishizaka Y, Ishizaka Y, Tanaka M, Kitamura K, Kangawa K, Minamino N, Matsuo H, and Eto T. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem Biophys Res Commun 200: 642-646, 1994. PMID: 8166740.
- Kato J, and Kitamura K. Bench-to-bedside pharmacology of adrenomedullin. Eur J Pharmacol 764: 140-148, 2015. PMID: 26144371.
- Kita T, Ashizuka S, Ohmiya N, Yamamoto T, Kanai T, Motoya S, Hirai F, Nakase H, Moriyama T, Nakamura M, Suzuki Y, Kanmura S, Kobayashi T, Ohi H, Nozaki R, Mitsuyama K, Yamamoto S, Inatsu H, Watanabe K, Hibi T, and Kitamura K. Adrenomedullin for steroid-resistant ulcerative colitis: a randomized, double-blind, placebo-controlled phase-2a clinical trial. J Gastroenterol 56: 147-157, 2021. PMID: 33140199.
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, and Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553-560, 1993. PMID: 22925672.
- Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, and Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun 194: 720-725, 1993. PMID: 7688224.
- Lopez J, Cuesta N, Cuttitta F, and Martinez A. Adrenomedullin in nonmammalian vertebrate pancreas: an immunocytochemical study. Gen Comp Endocrinol 115: 309-322, 1999. PMID: 10480982.
- Martinez-Herrero S, and Martinez A. Adrenomedullin regulates intestinal physiology and pathophysiology. Domest Anim Endocrinol 56 Suppl: S66-83, 2016. PMID: 27345325.
- Martinez A, Cuttitta F, and Teitelman G. Expression pattern for adrenomedullin during pancreatic development in the rat reveals a common precursor with other endocrine cell types. Cell Tissue Res 293: 95-100, 1998. PMID: 9634601.
- Martinez A, Kapas S, Miller MJ, Ward Y, and Cuttitta F. Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology 141: 406-411, 2000. PMID: 10614663.
- Martinez A, Weaver C, Lopez J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, and Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology 137: 2626-2632, 1996. PMID: 8641217.
- Mittra S, and Bourreau JP. Gs and Gi coupling of adrenomedullin in adult rat ventricular myocytes. Am J Physiol Heart Circ Physiol 290: H1842-1847, 2006. PMID: 16327020.
- Muff R, Born W, and Fischer JA. Adrenomedullin and related peptides: receptors and accessory proteins. Peptides 22: 1765-1772, 2001. PMID: 11754962.
- Mulder H, Ahren B, Karlsson S, and Sundler F. Adrenomedullin: localization in the gastrointestinal tract and effects on insulin secretion. Regul Pept 62: 107-112, 1996. PMID: 8795072.
- Onur OE, Guneysel O, Akoglu H, Denizbasi A, and Onur E. Adrenomedullin reduces the severity of cerulein-induced acute pancreatitis. Peptides 28: 2179-2183, 2007. PMID: 8795072.
- Sah RP, Nagpal SJ, Mukhopadhyay D, and Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 10: 423-433, 2013. PMID: 23528347.
- Schonauer R, Els-Heindl S, and Beck-Sickinger AG. Adrenomedullin - new perspectives of a potent peptide hormone. J Pept Sci 23: 472-485, 2017. PMID: 28150464.
- Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, and Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem 270: 4412-4417, 1995. PMID: 7876206.
- Tsuchida T, Ohnishi H, Tanaka Y, Mine T, and Fujita T. Inhibition of stimulated amylase secretion by adrenomedullin in rat pancreatic acini. Endocrinology 140: 865-870, 1999. PMID: 9927317.
- Tsuruda T, Kato J, Kuwasako K, and Kitamura K. Adrenomedullin: Continuing to explore cardioprotection. Peptides 111: 47-54, 2019. PMID: 29577955.
- Watkins HA, Chakravarthy M, Abhayawardana RS, Gingell JJ, Garelja M, Pardamwar M, McElhinney JM, Lathbridge A, Constantine A, Harris PW, Yuen TY, Brimble MA, Barwell J, Poyner DR, Woolley MJ, Conner AC, Pioszak AA, Reynolds CA, and Hay DL. Receptor Activity-modifying Proteins 2 and 3 Generate Adrenomedullin Receptor Subtypes with Distinct Molecular Properties. J Biol Chem 291: 11657-11675, 2016. PMID: 27013657.
- Wong HK, Tang F, Cheung TT, and Cheung BM. Adrenomedullin and diabetes. World J Diabetes 5: 364-371, 2014. PMID: 24936257.
- Zudaire E, Cuttitta F, and Martinez A. Regulation of pancreatic physiology by adrenomedullin and its binding protein. Regul Pept 112: 121-130, 2003. PMID: 12667633.