Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.32
| Attachment | Size |
|---|---|
| 157.09 KB |
1. Introduction
Over the past several decades, the importance of the stroma to cancer progression has become increasingly recognized. Rather than existing in isolation, cancer cells are surrounded by a tumor microenvironment that is largely the product of pancreatic stellate cells (PSC) that are sometimes referred to as stromal or cancer-associated fibroblasts. The tumor microenvironment also includes other cells such as immune cells and adipocytes as well as vascular and lymphatic networks and prominent extracellular matrix. In both chronic pancreatitis and in pancreatic adenocarcinoma (PAC) the stroma is problematic because it is quite extensive. In particular, the desmoplastic nature of PAC contributes to the limited surgical options for most patients at the time of diagnosis. Furthermore, recent evidence indicates that in PAC the stroma influences chemoresistance and metastasis.(2, 5)
Most in vivo studies of PAC use orthotopic or even in some cases subcutaneous models formed by injection of cancer cells only. Few such models develop stroma and therefore they do not fully simulate the relevant tumor microenvironment.(3) In order to better understand the role of the tumor microenvironment, our group and others have recently utilized co-injection orthotopic models of PAC which involves co-injection of PAC and PSCs.(2, 4) For this purpose, we isolated human PSCs from a resected surgical specimen of PAC. The PSCs have been used for in vitro and in vivo studies as primary cells, or after immortalization with SV40T and telomerase.(2) We obtained similar results with both primary and immortalized cells. However, the immortalized cells have the advantages that they are more stable, do not senesce, and can be manipulated (gene silencing or expression) for long-term studies such as in vivo experiments.
In addition, we use cancer cells labeled with firefly luciferase to allow monitoring of primary tumor growth and development of metastases in real time. This non-invasive imaging is extremely useful with orthotopic tumors which cannot be accurately measured by conventional means without sacrificing the animal.
In this chapter, the methods for using an orthotopic co-injection model of PAC will be outlined.
2. Materials
A. Cells
- Pancreatic cancer cells, labeled with luciferase. We have used cell lines obtained from American Type Culture Collection (ATCC, Manassas VA) or other sources. These were stably labeled with firefly luciferase using lentiviral transfection, which allows monitoring of tumor growth in real time with IVIS imaging.(1) Remember that no single cell model adequately represents PAC due to its inherent heterogeneity. Therefore, it is advisable to test several different cell lines.
- Human pancreatic stellate cells (immortalized or primary)(2)—these can be obtained from surgical specimens of PAC. In our hands, In vivo studies have been performed with primary cells or immortalized cells with similar results.
B. Animals
- Immune deficient mice- Several models are available. Nude mice are less expensive but the immune system is not as completely suppressed as SCID mice or NOD Scid mice. Therefore, tumors are slower to develop and to metastasize in Nude mice. In general, males are larger and thus may be easier to use for orthotopic models.
C. Bioluminescence imaging(1)
- IVIS imaging system (Caliper Life Sciences, Hopkinton MA) or similar systems
- D-Luciferin, Firefly, potassium salt, 1.0 g/vial (Caliper Life Sciences, Catalog #XR-1001)
- Dulbecco’s Phosphate Buffered Saline (DPBS), without Mg2+ and Ca2+
D. Surgical procedure
- Syringes—1-ml
- Needles—30-gauge
- Surgical instruments—scissors, forceps (smooth and serrated), wound clips and clip applier, cotton tipped applicators (Q-tips), gauze, 70% EtOH, heat lamp or other warmer, anesthetic
3. Methods
A. Prepare cell suspension
- Trypsinize both PSC and PAC cells. Count cells and confirm cell viability with trypan blue.
- Wash with Hank’s balanced salt solution (HBSS; Invitrogen) or PBS, 2X
- Spin down, re-suspend pellet in HBSS (or PBS) so that your cells to be injected are in 50 ul volume. Typically, we use 2.5 x 105 to 1x106 tumor cells per mouse. For co-injection, the PSCs are mixed with the PAC cells before injection. Ratios of PSC to PAC can be adjusted. We find that 1:1 PSC to PAC is sufficient to observe the influence of PSCs on tumor development.
- We usually prepare a total number of cells ~50% greater than what is needed, to allow for cell loss in the syringe, needle, etc.
- If you have many mice to inject, you might want to divide the cell suspension into several aliquots to try to ensure that the cells are evenly suspended in solution rather than all the cells pelleting in one vial at the bottom. Keep on ice.
- Make sure your cells are well-suspended in solution. Gently pass the cells through a 18-gauge needle a few times to re-suspend the cells, if needed, or vortex cells (very gently!) to keep cells well suspended.
- Use a 30-gauge needle and 1-ml syringe for injection.
B. Surgical procedure
- Anesthetize animal—our lab uses ketamine (100 mg/kg) + xylazine (2.5 mg/kg), intraperitoneal injection using 27-ga needle.
- Clean the left flank with alcohol wipe, or Betadine-soaked gauze.
- Identify ribs on left side and lift skin of left flank (subcostal region) with forceps and snip the skin and subcutaneous layer of muscle/peritoneum (0.5-1 cm length). To enter the abdominal cavity, you may need to open the skin and muscle/peritoneum with 2 cuts. This spot should be just inferior to the ribs, and slightly anterior.
- Identify the spleen. The pancreas is located just deep to the spleen, and looks thin and white, or very pale pink/tan. Don’t confuse the pancreas with mesenteric fat.
- With the left hand, pick up the tail of the pancreas with smooth forceps (not forceps with teeth as this will damage the pancreas).
- With the right hand, take a moistened Q-tip and roll it under the pancreas tail to “splay” out the pancreas.
- Use the left hand to gently grasp the tail of the pancreas with smooth forceps and stretch it out very gently towards yourself.
- Use the right hand to inject the cells slowly (50 ul volume) into the tail of the pancreas, just under the capsule. A wheal or bubble should form, just as in a subQ injection. Keep both hands steady to avoid leakage of the cells.
- Keep the needle in the pancreas until after you stop injecting. Then, pull the needle out slowly.
- Immediately press a dry Q-tip on the injection site to absorb any cells that have leaked. Hold pressure for a few seconds.
- Replace the pancreas and spleen into the peritoneal cavity.
- Wipe the inside of the incision site (in all directions) with a Q-tip moistened with 70% EtOH to minimize wound tumor formation.
- Close the wound with metal clips. Usually 2 clips should completely close the small incision. Be sure to close both the peritoneum/muscle and skin, otherwise a hernia will develop! If using metal clips, closing the 2 layers as one layer is fine, otherwise, if using sutures, they need to be closed separately.
- Post-procedure recovery: allow mouse to recover under a heat lamp or other warmer.
C. IVIS imaging
- Prepare a fresh stock solution of luciferin at 15mg/ml in DPBS. Inject 10 ul/g of body weight. Each mouse should receive 150 mg luciferin/kg body weight. (e.g. For a 10 g mouse, inject 100 ul to deliver 1.5 mg of luciferin.)
- Inject the luciferin intra-peritoneally (i.p.) 10-15 minutes before imaging*.
* A luciferin kinetic study should be performed for each animal model to determine peak signal time.
4. Results
Using this model, we showed that co-injection of PSCs resulted in increased growth of the primary pancreatic tumor and metastases.(2) The influence of PSCs was dose-dependent since a tumor:stroma ratio of 1:5 resulted in greater tumor growth than a ratio of 1:1 (Figure 1). Furthermore, the presence of PSCs increased the incidence of tumor formation when limiting numbers of cancer cells were injected. Thus, PSCs may also be involved in tumor initiation as well as tumor progression.
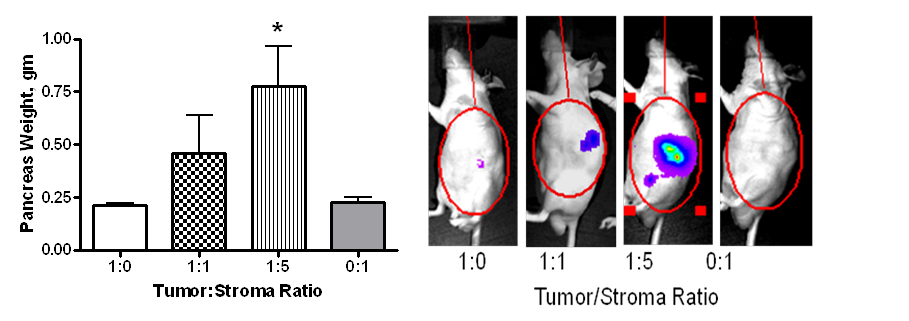
Figure 1. Co-injection of PSCs with Bxpc3 tumor cells labeled with firefly luciferase in an orthotopic nude mouse model of pancreatic cancer results in increased tumor growth in a dose-dependent manner (left panel).(2) Tumor progression was monitored in real-time using IVIS luciferase imaging (right panel).(2)
There are limitations to this model. One limitation is that despite the inclusion of the PSCs, in our experiments using Bxpc3 cancer cells, there was no significant increase in the level of stroma developed in the tumors formed by co-injection with PSCs compared to tumors with cancer cells alone. This observation suggested that the co-injected PSCs did not persist through the full period of the studies, which was subsequently confirmed (unpublished observation). Nevertheless, in a model using AsPC-1 cancer cells co-injected with PSCs, increased collagen deposition was observed in the tumors, confirming that the injected PSCs influence stromal development.(5)
5. References
- Arumugam T, Simeone DM, Van Golen K, and Logsdon CD. S100P promotes pancreatic cancer growth, survival and invasion. Clin Cancer Res 11: 5356-5364, 2005. PMID: 16061848
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, and Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68: 918-926, 2008. PMID: 18245495
- Killion JJ, Radinsky R, and Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer and Metastasis Reviews 17: 279-284, 1998. PMID: 10352881
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, and Apte MV. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68: 2085-2093, 2008. PMID: 18381413
- Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS, and Apte MV. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathology 177: 2585-2596, 2010. PMID: 20934972


