Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2012.2
| Attachment | Size |
|---|---|
| 339.11 KB |
1. Introduction
The pathogenesis of acute pancreatitis involves the interplay of local and systemic immune responses that are often difficult to characterize. There is an intricate balance between localized tissue damage with the systemic production of pro-inflammatory and anti-inflammatory mediators. To study the pathophysiological changes occurring during acute pancreatitis in animal models, we have employed several methods to quantitate various markers of inflammation. This entry presents the methods that we used to determine the levels of myeloperoxidase activity, chemokines and adhesion molecules protein, substance P and hydrogen sulfide (main interests of our laboratory), and pulmonary microvascular permeability.
2. Caerulein-Induced Pancreatitis in Mouse
Caerulein, a cholecystokinin-pancreozymin analogue, has been widely used for the induction of acute pancreatitis in laboratory animals (12,13,19,25). Caerulein administration causes intracellular proteolytic enzyme activation resulting in pancreatic acinar autolysis. Elevation in the concentrations of plasma pancreatic enzymes, acinar cell necrosis and pancreatic inflammation are the common observations. This model is also useful for investigating the acute pancreatitis associated lung injury (9), which resembles the early stages of the acute respiratory distress syndrome in humans. This is a relatively simple and inexpensive model, and has been frequently used to study the pathophysiological events in acute pancreatitis.
2.1 Materials
- Male Balb/C mice, 10-12 weeks old (25 to 35 g)
- 1 mL syringes
- 27G needles
- Caerulein (Bachem AG, Switzerland)
- Pentabarbitone solium (Nembutal ®, Ceva Chemicals, Australia)
2.2 Methods
1. All experiments are performed in accordance with established International Guiding Principles for Animal Research. The protocol discussed below was approved by Animal Research Ethics Committee, National University of Singapore. The senior author (MB) has also worked with caerulein-induced model of acute pancreatitis following protocols approved by Institutional Animal Care and Use Committee, Beth Israel Deaconess Medical Center, Boston, MA, and United Kingdom Home Office.
2. Male Balb/C mice weighing between 25 to 35 g are housed under climate-controlled conditions with a 12-hour alternate light/dark cycle and fed standard laboratory chow and allowed to drink water ad libitum throughout the experiment.
3. Mice are randomly assigned to control or experimental groups (n=8 to 10). Caerulein is injected intraperitoneal. Each mouse receives 10 consecutive hourly doses of caerulein (50 μg/kg). The 10 hourly-injection group represents the severe form of AP, in which secondary lung injury develops.
4. One hour after the last caerulein injection animals are sacrificed by an intraperitoneal injection of a lethal dose of pentabarbitone sodium Nembutal® (90 mg/kg, three times the dose for surgical anesthesia).
5. Blood samples are collected by cardiac puncture into heparinised syringes and centrifuged at 2,000 × g for 10 minutes at 4°C to obtain plasma. Fractions of approximately 40 mg of the tissues of interest (pancreas or lung) are rapidly isolated, frozen in liquid nitrogen and kept at -70°C until use.
3. Determination of Myeloperoxidase Activity
Neutrophil sequestration in inflamed tissues is quantified by measuring the tissue myeloperoxidase (MPO) activity (14,22). MPO is a hemoprotein that is stored in azurophilic granules of polymorphonuclear neutrophils and macrophages. MPO catalyzes the conversion of chloride and hydrogen peroxide to hypochlorite and is secreted by activate neutrophils during inflammatory condition. It is characterized by strong oxidative and proinflammatory properties.
3.1 Materials
- Refrigerated centrifuge (capable of up to 13,000 × g)
- Polytron tissue homogenizer
- Fluorescence spectrophotometer plate reader. We use a Tecan plate reader, but any plate reader will work.
- Probe type sonicator
- Water Bath (37°C)
- Chemicals:
- 20 mM sodium phosphate buffer (pH 7.4)
- 50 mM sodium phosphate buffer (pH 6.0)
- 80 mM sodium phosphate buffer (pH 5.4)
- 0.5%w/v hexadecylmethylammonium bromide (Cat #: H5882, Sigma)
- 3,3',5,5'-tetramethylbenzidine (TMB) liquid substrate system for ELISA (Cat #: T0440, Sigma)
- 2N sulfuric acid (H2SO4)
- 1% Hoechst dye 33256 (Sigma)
- Salmon Testis DNA standards
3.2 Methods
1. Pancreas or lung tissue sample initially stored at -70°C is thawed at 4ºC. The tissue is then homogenized in 1 mL of 20 mM sodium phosphate buffer (pH 7.4) using a Polytron homogenizer at 10,000 rpm.
2. The homogenate is centrifuged at 13,000 × g for 10 min at 4°C. The resulting pellet is resuspended (gently perturbing the pellet with the pipette tip followed by 8 to 10 pipetting strokes) in 1 mL of a solution comprising of 50 mM sodium phosphate buffer (pH 6.0), 0.5% hexadecylmethylammonium bromide.
3. The resultant suspension is subjected to four freeze-thaw cycles and then sonicated for 40 sec on ice (20 kHz, 500 W). This is followed by centrifugation at 13,000 × g for 10 min at 4°C to acquire the supernatant for MPO assay.
4. 50 µL of extracted enzyme is mixed with 50 µL of TMB liquid substrate system for ELISA and incubated at 37°C for 2 min.
5. An equal volume of 2N H2SO4 is added to stop the reaction. The absorbance at 405 nm is measured using the Tecan spectrophotometry plate reader. This absorbance is then corrected for the DNA content of the respective samples and results expressed as fold increase over control group.
6. The determination of the DNA content of the sample crude homogenates (obtained after step 1 above) is performed fluorometrically using Hoechst dye 33256 as originally described (18). 10 µL of samples or standards (0 to 0.4 µg/ml) are allowed to incubate with 90 µL of 1% Hoechst dye solution for 10 min in the dark and the flourescence is read on the Tecan spectrofluorometry plate reader (excitationλ = 360 nm, emissionλ = 450 nm).
7. A standard curve constructed by using salmon testis DNA is plotted using Microsoft Excel and the concentration of DNA in the samples calculated by the linear equation obtained using linear regression.
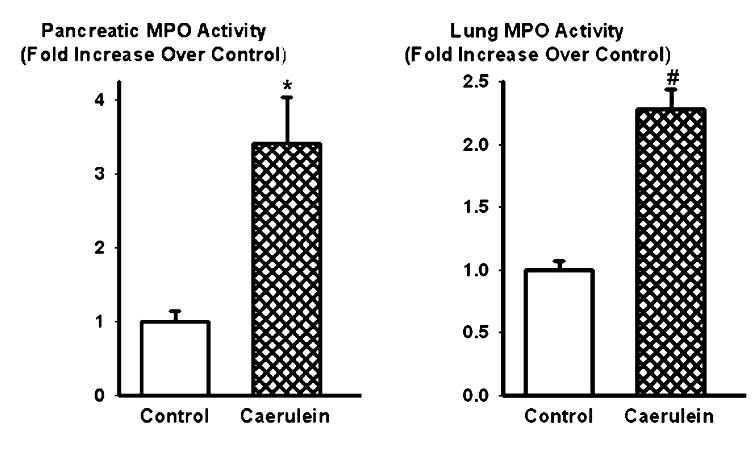
Fig 1. Effect of caerulein (50 µg/kg/hr, i.p., 10 injections) on MPO enzyme activity in the pancreas (left) and lungs (right). Histograms show mean values ± SEM (n=8). * indicates significantly different from the control group (pancreas, P < 0.05); # indicates significantly different from the control group (lungs, P < 0.05). Modified from Reference 20.
4. Determination of Chemokines and Adhesion Molecules
The recruitment of inflammatory cells, such as neutrophils, monocytes and macrophages, to the sites of inflammation is mediated by various chemokines and adhesion molecules (2,23). The release of chemokines and the interaction between adhesion molecules on the endothelial cells and leukocytes result in a series of events that bring about extravasation of leukocytes from the circulation. Chemokines are a family of small proteins secreted by cells to act as a chemoattractant to guide the migration of nearby responsive cells. During inflammation, a number of chemokines are release to recruit various inflammatory cells to the site of injury. On the other hand, adhesion molecules are proteins located on the cell surface involved in the binding with other cells in the process called cell adhesion. These proteins are typically transmembrane receptors.
4.1 Materials
- Refrigerated centrifuge (up to 13,000 × g)
- Polytron tissue homogenizer
- Microplate shaker
- Fluorescence spectrophotometer plate reader
- Duoset ELISA kit (R&D Systems, Minneapolis, MN)
- 2N sulfuric acid (H2SO4)
- 10 mM sodium phosphate buffer (pH 7.4)
4.2 Methods
1. Harvested pancreas and lung samples are thawed and homogenized in ice-cold 10 mM phosphate buffer (pH 7.4) using a polytron homogenizer at 10,000 rpm.
2. The homogenates are centrifuged at 2,000 × g for 15 min at 4°C. The supernatants are then collected for detection of chemokines/adhesion molecules, using ELISA kit (Duoset ELISA kit, R&D Systems, Minneapolis, MN).
3. To determine chemokines/adhesion molecules concentration we use the appropriate Duoset ELISA kit. Anti-X primary antibody (X: chemokine or adhesion molecule of interest) is aliquoted onto ELISA plates as directed in the kit and incubated at 4°C overnight.
4. The wells are then washed three times with washing buffer, followed by 1 h incubation with blocking buffer at room temperature.
5. The wells are then washed three times with washing buffer. 100 uL of chemokine/adhesion molecules standards or unknown samples (in duplicates) are added into the wells and incubated on a microplate shaker for 2 h.
6. The plates are washed three times with washing buffer and 100 µL of biotinylated anti-X antibody (X: chemokine or adhesion molecule of interest) is added and incubated on a microplate shaker for 2 h.
7. Plates are again washed three times with washing buffer and. 100 µL of streptavidin bound horseradish peroxidase (HRP) is added and incubated for 20 min.
8. This is followed by another round of three washes using washing buffer. Finally, 100 µL of tetramethylbenzidine solution (Substrate Solution, R&D Systems) is then added to each well and incubated for 20 min at room temperature, protected from light.
9. The reaction is stopped by the addition of 50 µL 2N H2SO4 (mixed well on a microplate shaker). Absorbance at 450 nm is measured within 15 min.
10. The DNA contents of the unknown samples (crude homogenates obtained after step 1 above) are also determined as described in section 3.2.6 and the final results expressed as pg/µg DNA in tissue samples.
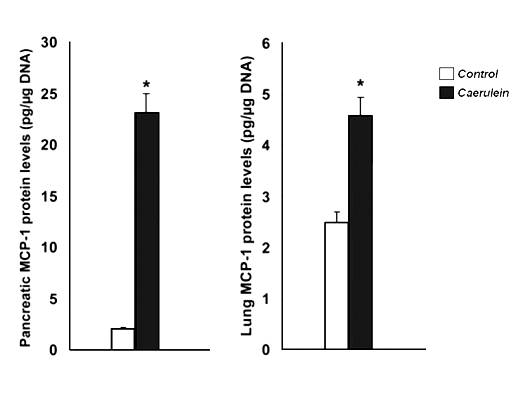
Fig 2. Effect of caerulein (50 µg/kg/hr, i.p., 10 injections) on monocyte chemotactic protein (MCP)-1 concentration in the pancreas (left) and lungs (right). Histograms show mean values ± SEM (n=8). * indicates significantly different from the control group (P < 0.05). Modified from Reference 24.
5. Determination of Substance P Levels
Substance P (SP) acts as a pro-inflammatory mediator in many inflammatory states including asthma, immune-complex-mediated lung injury, experimental arthritis, and inflammatory bowel syndrome (IBS) (3-8). Knockout mice deficient in NK1R and in the PPT-A gene are protected against pancreatitis and associated lung injury (9,10,16). These findings suggest an important pro-inflammatory role for SP and NK1R in acute pancreatitis and associated lung injury. The quantitation of SP in the tissue may be performed using ELISA.
5.1 Materials
- Refrigerated centrifuge (up to 13,000 × g)
- Polytron tissue homogenizer
- Freeze-dryer
- Fluorescence spectrophotometer
- C18 Sep-Pak SPE cartridge columns (Waters, Cambridge, MA)
- Substance P EIA (Enzyme immunoassay) Kit (Cat #: S-1180, Bachem AG, Switzerland)
- Chemicals
- Acetonitrile
- Methanol
- 0.1% tri-fluoroacetic acid (TFA)
- 0.2% tri-fluoroacetic acid (TFA)
- 10 nM sodium ethylenediaminetetraacetate (EDTA)
- 150 mM sodium chloride (NaCl)
- 50 mM sodium phosphate buffer (pH 7.0)
- Substance P (as standards, Cat #: H-1890, Bachem AG, Switzerland)
5.2 Methods
1. Sep-Pak C18 cartridge columns are washed with 10 mL methanol using a 10 mL syringe, followed by 10 mL of 0.1% TFA in water.
2. Tissue samples (pancreas or lungs) taken from in vivo studies are homogenized in 1.0 mL ice-cold assay buffer (50 mM sodium phosphate buffer, pH 7.0; 150 mM NaCl and 10 nM sodium EDTA) for 20 sec. The homogenates are centrifuged (13,000 × g, 20 min at 4°C) and the supernatants collected.
3. The supernatants obtained are then diluted 1:1 with 0.2% TFA (in water) and loaded onto the C18 cartridges columns and allowed to flow through slowly by gravity. The columns are then washed with 10 mL 0.1% TFA (in water).
4. The peptides absorbed on Sep-Pak C18 cartridge columns are eluted with 1.5 mL 75% v/v acetonitrile in 0.1%: 25% TFA (0.1% in water).
5. The eluents are freeze-dried and reconstituted in 0.5 mL assay buffer (step 2 above).
6. SP content is determined using an ELISA kit (Bachem) according to the manufacturer’s instructions and expressed as ng/μg DNA. SP can be measured in the range from 0 to 10 ng/mL in this assay.
7. The values are then corrected for the DNA content (using crude homogenates obtained in step 2, before centrifugation, above) as described in section 3.2.6 and results expressed as fold increase over control group.
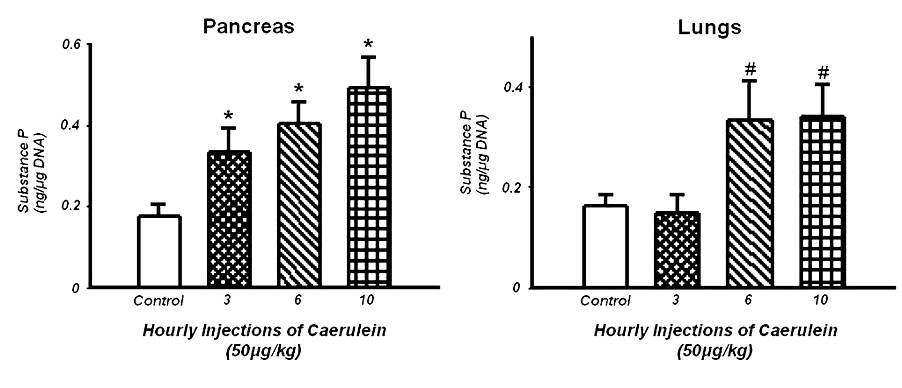
Fig 3. Effect of caerulein (50 µg/kg/hr, i.p., 10 injections) on SP concentration in the pancreas (left) and lungs (right). Histograms show mean values ± SEM (n=10). * indicates statistically significantly differences from the control group (pancreas, P < 0.05); # indicates statistically significantly differences from the control group (lungs, P < 0.05). Modified from Reference 20.
6. Determination of Hydrogen Sulfide Levels and Hydrogen Sulfide Synthesizing Enzyme Activity
Hydrogen sulphide (H2S) is a gaseous messenger that is endogenously synthesized from L-cysteine by pyridoxal-5'-phosphate-dependent enzymes, cystathionine β-synthetase and cystathionine γ-lyase (1). Recent studies have demonstrated the key roles of H2S in the process of inflammation (26). Therefore, it is valuable to determine the concentration of H2S and H2S synthesizing enzyme activity using this simple method.
6.1 Materials
- Refrigerated centrifuge (up to 13,000 × g)
- Polytron tissue homogenizer
- Spectrophotometer
- Shaking water bath (37°C)
- Chemical fume hood
- Chemicals
- 0.9% w/v sodium chloride (normal saline)
- 100 mM potassium phosphate buffer (pH 7.4)
- 10% w/v trichloroacetic acid (TCA)
- 1% w/v zinc acetate
- 20 µM N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl
- 30 µM Ferric chloride (FeCl3) in 1.2M HCl
- 100 µM Sodium hydrosulfide (NaHS, as standard) in water
- 10 mM L-cysteine
- 2 mM pyridoxyal-5’-phosphate
6.2 Methods
A. Determination of Hydrogen Sulphide Levels
1. Blood samples collected are centrifuged at 5,000 × g for 10 min at 4°C to obtain the clear plasma samples. Tissue samples (pancreas or lungs) taken from in vivo studies are homogenized in 10 volumes (1 g/10 mL) ice-cold 100 mM potassium phosphate buffer (pH 7.4) for 20 sec.
2. The reaction mixture consists of 120 µL plasma (or pancreas/lung homogenate), 100 µL water, 120 µL TCA (10% w/v), 60 µL zinc acetate (1% w/v), 40 µL N,N-dimethyl-p-phenylenediamine sulfate (20 µM in 7.2 M HCl) and 40 µL FeCl3 (30 µM in 1.2 M HCl). It is allowed to stand for 10 min at room temperature. All reactions are performed in a chemical fume hood.
(Note 1: The basis of the assay: H2S produced in the incubate reacts with zinc acetate to form zinc sulphide. Zinc sulphide dissolves in a hydrochloride acid solution of N,N-dimethyl-p-phenylenediamine sulphate to yield methylene blue in the presence of ferric chloride. The concentration of the final product can be quantitated spectrophotometrically at 670 nm.)
3. The absorbance of the resulting solution is measured at 670 nm. H2S is calculated against a calibration curve generated using NaHS (ranging from 3.125 to 100 µM).
4. The absorbance is corrected for the DNA content of tissue sample (using plasma or crude homogenates obtained in step 1 above) as described in section 3.6.6.
B. Determination of H2S Synthesizing Enzyme Activity:
1. To determine H2S synthesizing enzyme activity, the enzyme reaction is carried out with the substrate cysteine in the presence of pyridoxal phosphate, the co-factor.
2. Samples obtained from step 1 are used. The reaction mixture consists of 10 μl L-cysteine (10 mM), 10 μl pyridoxyal 5´-phosphate (2 mM), 15 μl normal saline and 215 μl tissue homogenate (total reaction volume is 250 μl, prepared on ice).
3. The reaction is performed in tightly stoppered cryovial test tubes and initiated by transferring the tubes from ice to a shaking water bath at 37ºC.
4. After 30 min incubation, 125 μl of 1% w/v zinc acetate is added to trap evolved H2S. This is followed by the addition of 125 μl of 10% v/v trichloroacetic acid to denature the protein and to stop the reaction.
5. 65 μl of N,N-dimethyl-p-phenylenediamine sulphate (20 μM, in 7.2 M HCl) is added, immediately followed by 65 μl of FeCl3 (30 μM in 1.2 M HCl).
6. The absorbance of the resulting solution at 670 nm as described in step 3 and 4, above. H2S synthesizing enzyme activity is calculated by dividing the H2S concentration by DNA content and 30 minutes, and expressed in µM H2S/mg DNA/min at 37ºC.
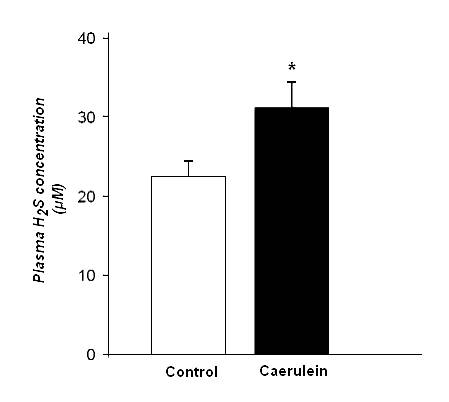
Fig 4. Effect of caerulein (50 µg/kg/hr, i.p., 10 injections) on H2S concentration in the plasma. Histograms show mean values ± SEM (n=10). * indicates statistically significant differences from the control group (P < 0.05). Modified from Reference 11.
7. Determination of Pulmonary Microvascular Permeability
Central to an intact alveolar-capillary barrier is the dynamic rearrangement of the cytoskeleton. This reorganization of the cytoskeleton is crucial to control passive transport and active movement of solutes across the tenuous alveolar-capillary arrangement (15). During the initial stages of pulmonary inflammation, stress fibers form within the cells arranging the alveolar-capillary barrier resulting in increased pulmonary microvacular permeability (17,21,27,28). This results in tissue edema, reduction in oxygen exchange, infiltration of inflammatory cells and compromised pulmonary function. The degree of pulmonary microvascular permeability may be used to determine the severity of pulmonary injury.
7.1 Materials
- Refrigerated centrifuge (up to 3,000 × g)
- Surgery instruments
- 4-0 suture
- 1 mL syringes, 23G and 27G needles
- Flexible Polyethylene tubing PE-50
- Fluorescence spectrophotometer
- 2.5 mg/mL FITC-albumin (Cat #: A9771, Sigma)
- 0.9% w/v sodium chloride (normal saline)
7.2 Methods
1. Two hours before sacrifice, each mouse is given an intravenous bolus injection containing FITC-albumin (5 mg/kg @ 2 mL/kg in normal saline) via the tail vein using a 27G needle.
2. A 23G needle is connected to a 5 cm long PE50 (polyethylene) tubing and attached onto a syringe pre-filled with 1mL normal saline.
3. Immediately after sacrifice, the trachea is exposed. A small cut is made and the PE50 tubing is inserted 1 cm towards the lungs. A ligature is placed just below the cut to secure the tubing and prevent leakage of lavage fluid.
4. The lungs are lavaged three times with 1 mL of normal saline. The lavage fluid is combined.
5. Whole blood is collected by cardiac puncture into a heparinized syringe and a plasma sample is obtained by centrifugation at 3,000 × g for 10 min at 4°C.
6. FITC fluorescence is measured in the lavage fluid and plasma (excitation = 494 nm; emission = 520 nm). The ratio of fluorescence emission in lavage fluid to plasma is calculated and used as a measure of pulmonary microvascular permeability.
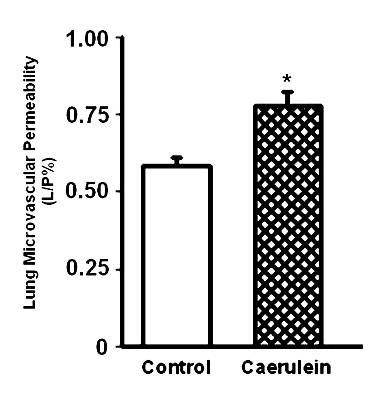
Fig 5. Effect of caerulein (50 µg/kg/hr, i.p., 10 injections) on pulmonary microvascular permeability in the lungs. Histograms show mean values ± SEM (n=10). * indicates significantly different from the control group (P < 0.05). Modified from Reference 20.
8. References
- Ang AD, Ramasamy T, Bhatia M. Hydrogen sulphide. The Pancreapedia: Exocrine Pancreas Knowledgebase, 2011. DOI: 10.3998/panc.2011.18
- Baggiolini M, Dewald B, Moser B. Human chemokines: An update. Annu Rev Immunol. 15: 675-706, 1997. PMID: 9143704
- Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy 1: 343–351, 2002. PMID: 14561181
- Bhatia M. Tachykinins as therapeutic targets in inflammation. Curr Med Chem—Anti-Infl Anti-Allergy Drugs 2: 19–27, 2003. DOI:10.2174/1568014033355862
- Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome—an update. Med Chem Rev Online 1: 25–26, 2004. DOI:10.2174/1567203043480395
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 190: 117–125, 2000. PMID: 10657008
- Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 202: 145–156, 2004. PMID: 14743496
- Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs 2: 496–501, 2001. PMID: 11566005
- Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA 95: 4760–4765, 1998. PMID: 9539812
- Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 284: G830–G836, 2003. PMID: 12684214
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 19: 623-625, 2005. PMID: 15671155
- Bieger W, Martin-Achard A, Bassler M, Kern HF. Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. IV. Stimulation by in vivo infusion of caerulein. Cell Tissue Res. 165: 435-453, 1976. PMID: 1260840
- Bieger W, Seybold J, Kern HF. Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. V. Kinetic studies on accelerated transport following caerulein infusion in vivo. Cell Tissue Res. 170: 203-219, 1976. PMID: 954054
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 78: 206–209, 1982. PMID: 6276474
- Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 16: 583-585, 2002. PMID: 11919161
- Grady EF, Yoshimi SK, Maa J, Valeroso D, Vartanian RK, Rahim S, Kim EH, Gerard C, Gerard N, Bunnett NW, Kirkwood KS. Substance P mediates inflammatory oedema in acute pancreatitis via activation of the neurokinin-1 receptor in rats and mice. Br J Pharmacol. 130: 505–512, 2000. PMID: 10821777
- Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem. 277: 42596-42602, 2002. PMID: 12202485
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 102: 344-352, 1980. PMID: 6158890
- Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Path Anat and Histol. 373: 97-117, 1977. PMID: 139754
- Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 327: 509-515, 2005. PMID: 15629143
- Minamino T, Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest. 116: 2316-2319, 2006. PMID: 16955131
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14: 157-167, 1985. PMID: 2997548
- Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 114: 1066-1090, 1998. PMID: 9558298
- Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 292; G143-G153, 2007. PMID: 16873893
- Tardini A, Anversa P, Bordi C, Bertaccini G, Impicciatore M. Ultrastructural and biochemical changes after marked caerulein stimulation of the exocrine pancreas in the dog. Am J Pathol. 62: 35-56, 1971. PMID: 5538718
- Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin. Pharmacol. 4: 13–32, 2011. PMID: 22115346
- Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 288: L749-L760, 2005. PMID: 15591411
- Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 290: L1173-L1182, 2006. PMID: 16428270


