Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.11
| Attachment | Size |
|---|---|
| 287.2 KB |
In 1995, an Editorial in Gastroenterology stated: “… because of differences in relative abundance and ease of study, ductal cells have received less scrutiny than acinar cells… One way to overcome the problems inherent with studies of acutely isolated duct cells is to culture them in vitro… However, whereas monolayers of duct cells in culture have been produced, functional studies have not yet been successful” (7). Since that Editorial, we have been able to culture and propagate non-transformed and well-differentiated pancreatic duct epithelial cells (PDEC), obtained from the main pancreatic duct of a dog, and have used them as model for pancreatic ductal secretion.
In this chapter, we will a) describe how these cells were originally isolated, b) detail methods for their culture, and c) summarize the published secretory studies that used these cells as a model.
A. Isolation of Dog PDEC and Human Gallbladder Myofibroblasts (14-16)
1. Materials
- Main pancreatic duct from a dog
- 0.5% trypsin/ 0.2% EDTA (Sigma T4174, St. Louis, MO)
- Bronchial epithelial growth medium (Clonetics Corp., San Diego, CA)
- Eagle’s Miminum Essential Medium (MEM) (Gibco 11090-081, Carlsbad, CA)
- Phosphate buffered saline, pH 7.4 (PBS)
- Vitrogen (previously from Celtrix Labs, Palo Alto, CA, now remarketed as PureCol, Advanced Biomatrix, San Diego, CA)
- 60 mm culture plates (Becton Dickinson. Franklin Lakes, NJ)
- Transwell inserts (3 µm pore size, 24 mm diameter, Costar 3414)
2. Methods
- Dog PDEC.
The dog PDEC were originally generated by Oda et al. as follows (14-16). The pancreas was removed from a normal mongrel dog, the accessory (main) pancreatic duct dissected, a 1 cm segment of the duct was cut open longitudinally to display the luminal mucosa, and epithelial cells were dissociated from the mucosa through treatment with 0.25% trypsin/ 0.1% EDTA in PBS for 30 min at 37oC. The detached cells were then harvested by centrifugation at 500 x g for 5 min, washed three times in PBS, seeded onto Vitrogen-coated plates (1:1 mixture of Vitrogen and medium), and cultured in low-calcium, serum-free bronchial epithelium growth medium for a week to inhibit fibroblast growth. These cells were subsequently transferred to Vitrogen-coated Transwell inserts (0.5 ml of diluted Vitrogen per insert, see next section for more details), and the inserts were suspended above a confluent feeder layer of cultured human gallbladder myofibroblasts. - Human Gallbladder Myofibroblasts.
Cultured human gallbladder myofibroblasts (HGBMF) are used as a feeder layer, providing factors (yet uncharacterized) that support the growth and differentiation of the dog PDEC. They were originally isolated by Kuver et al.(6), by treating a normal human gallbladder with 0.5% trypsin / 0.2% EDTA for 1 hour to dissociate myofibroblasts (as well as mucosal epithelial cells). Because gallbladder myofibroblasts attach and grow on plastic culture plates while epithelial cells do not, HGBMF were selected by culturing them on 60 mm plastic culture plates (seeding density ~ 105 cells per dish) in MEM. The MEM was replaced every 2 days until HGBMF were confluent (usually after 1 week), at which time the cells were detached from the plates with 0.25% trypsin / 0.1% EDTA, plated onto 35 mm six-well plates at a density of 105 cells per well, and cultured in MEM.
B. Propagation and Maintenance of Dog PDEC
1. Materials:
- Cultured dog PDEC
- Cultured human gallbladder myofibroblasts
- 0.25% trypsin/0.1% EDTA
- Supplemented MEM:
- Eagle’s Minimum Essential Medium (MEM)
- 10% fetal bovine serum (Gibco 16000)
- 2mM L-glutamine (Sigma G5792)
- 20mM HEPES (Sigma H3375)
- 100 IU/ml penicillin
- 10 ug/ml streptomycin
- ITS (5µg/ml bovine insulin, 5µg/ml human transferrin, 5ng/ml sodium selenite) (Sigma I1884)
- Vitrogen (or PureCol, Advanced Biomatrix)
- Transwell inserts
- Six-well culture plates (Becton Dickinson, Franklin Lakes, NJ)
2. Methods:
- Plating human gallbladder myofibroblast cells (HGBMF).
Because HGBMF serve as a feeder layer for dog PDEC, they need to be prepared about 1 week beforehand. Stock cultures of HGBMF can be maintained in 60 mm culture plates (or T75 flasks). One confluent 60 mm plate (or T75 flask) can be used to seed up to 24 wells of 6-well plates plus one 60 mm stock plate (or T75 flask). Confluent HGBMF are washed with PBS (warmed to 37oC) and treated with 2 ml of 0.25% trypsin/ 0.1% EDTA (in PBS) at 37oC for 30 min. The detached cells, harvested in an additional 10 ml of supplemented MEM and collected after centrifugation at 500 x g for 5 min, are resuspended in 60 ml of supplemented MEM. The cell suspension is transferred to 6-well plates (2 ml per well) and the stock culture 60 mm plate (12 ml) and incubated at 37oC in 5% CO2/95% air. When the HGBMF are confluent (between 7-10 days), they can be used as a feeder layer for the PDEC grown on the Transwell or Snapwell inserts that will be suspended over them.Up to 10 passages of HGBMF can be used in this manner. Alternatively, normal human dermal myofibroblasts (Clonetics, San Diego, CA) can also be used in lieu of HGBMF (18); however, PDEC grown with human dermal myofibroblasts have not been as extensively characterized as PDEC grown with HGBMF.
- Coating 6-well plates or Transwell inserts with Vitrogen.
Vitrogen and MEM, cooled on ice for 10 min, are mixed in a 1:1 ratio and the resulting mixture is spread evenly to coat individual Transwell inserts (0.5 ml per insert). This Vitrogen coating is then allowed to solidify undisturbed in an incubator for 15 min at 37oC. - Trypsinization and seeding of cultured PDEC.
PDEC previously grown to confluence on Transwell membranes are washed with PBS at 37oC, incubated for 30 min with 2 ml 0.25% trypsin/ 0.1% EDTA at 37oC, harvested using 10 ml of supplemented MEM, and collected by centrifugation at 500 x g for 5 min. Cells harvested from one Transwell insert can be plated onto 6 to 12 new Vitrogen-coated Transwells. Supplemented MEM is added to the inside (top) of the Transwell insert (2 ml) as well as the outside (bottom) compartment (2 ml). These PDEC are then cultured at 37oC in 5% CO2/ 95% air. The cells are fed with supplemented MEM twice a week with 2 ml each in the top and bottom compartment.
As shown in Figure 1, in this culture system, a confluent feeder layer of HGBMF is maintained at the bottom of the well. When the dog PDEC are confluent, the media in contact with the top of the HGBMF feeder layer and the bottom of the Transwell insert, adjoining the basolateral membrane (BLM) of the PDEC, corresponds to the serosal compartment for PDEC. This shared compartment allows growth factors from the HGBMF to reach the PDEC. Conversely, the medium within the insert, overlying the apical membrane of PDEC will correspond to the luminal compartment for these cells.Prepared in this manner, dog PDEC can be propagated for > 40 passages and can be frozen (1 ml vials, 10% DMSO in supplemented MEM, MrFrosty freezing containers) and revived as necessary. Their characteristics and their use as models for PDEC physiology are summarized in the next section.
We have also tried to culture human PDEC in a similar manner. However, human PDEC could not be propagated beyond 10 passages and therefore we have not been able to characterize their secretory functions.
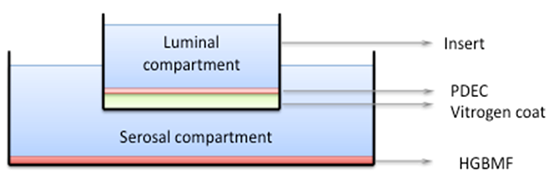
Figure 1: Culture system for dog pancreatic duct epithelial cells (PDEC). PDEC are cultured over the Vitrogen-coated permeable membrane at the bottom of a Transwell or Snapwell insert that is suspended over a feeder layer of human gallbladder myofibroblasts (HGBMF). When the PDEC monolayer is confluent, the compartment within the insert, overlying the PDEC corresponds to the luminal compartment while the compartment below the insert and PDEC corresponds to the serosal compartment. The serosal compartment allows factors secreted by HGBMF to reach the PDEC.
C. Cultured Dog PDEC Monolayers as Model for PDEC Studies
1. Characterization of cultured dog PDEC monolayers:
- Differentiation of cultured dog PDEC (16).
As shown in Figure 2, on electron microscopy, these PDEC are morphologically well-differentiated and grow as polarized cell monolayers. They display distinct apical brush border as well as basolateral membranes, tight junctions that will form a barrier between these surfaces, and intracellular mucin secretory granules. By immunochemistry, they only express markers for epithelial cells as well as carbonic anhydrase (to convert carbon dioxide to bicarbonate). Confluent cell monolayers are able to generate a transepithelial resistance of up to 1 kW.cm2. - Ion channels on cultured dog PDEC (2, 8, 10).
- Cl- channels
Using 125I- efflux studies, patch clamp measurements, and western blots, we demonstrated that these PDEC express, as expected, two distinct Cl- channels: a) CFTR, stimulated by forskolin and the cAMP pathway, inhibited by 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) and diphenylamine-2-carboxylic acid (DPC), but not 4,4'-diisothiocyanatostilbene-2,2'-disulfonate (DIDS), with linear I-V relationship, and immunoreactive by western blot, and b) an outward rectifying Ca2+-activated Cl- channel, stimulated by the Ca2+ ionophore A23187 and inhibited by NPPB, DPC, and DIDS. - K+ channels
Through 86Rb+ efflux studies, we demonstrated Ca2+-activated K+ channels that were stimulated by A23187, thapsigargin, and 1-ethyl-2-benzimidazolinone (1-EBIO), but not forskolin), and were inhibited by BaCl2, charybdotoxin, clotrimazole, and quinidine (but not 4-aminopyridine, apamin, tetraethylammonium, or iberiotoxin). These channels are located on the BLM of the PDEC, mediating an efflux into the serosal compartment. In Ussing chamber studies of apically-permeabilized PDEC monolayers (Fig. 3), these basolateral K+ channels mediated a charybdotoxin-sensitive increase in short-circuit current (Isc) stimulated by A23187 and 1-EBIO.Through patch clamp studies combined with calcium fluorometry, we demonstrated that these channels are of intermediate conductance (~50 pS), exhibit a K1/2 of ~ 0.5 mM, are activated [Ca2+]i increases and oscillations, and hyperpolarize the plasma membrane of PDEC.c. Exocytosis in PDEC (5).
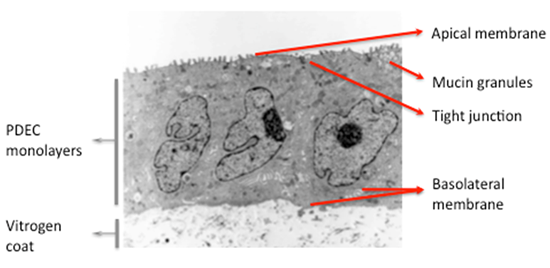
Figure 2: Electron photomicrograph of cultured dog PDEC (adapted from Oda et al. [16]). Culture dog PDEC grow as well differentiated, polarized cell monolayers on electron microscopy, exhibiting an apical membrane with microvilli, tight junctions, and a basolateral membrane. Intracellular secretory granules are also observed.
- Cl- channels
- Exocytosis from single, non polarized dog PDEC, cultured in HGBMF-conditioned medium and on glass slides coated with Vitrogen, was demonstrated by loading these cells with dopamine and using carbon-fiber amperometry to detect its oxidation once it is released from PDEC via exocytosis. Similar to endocrine cells and neurons, the quantal amperometric event that reflects a single vesicular fusion corresponding to a single exocytotic occurrence, exhibited a rapid onset and decay. This signal is also frequently preceded by a “foot”, which reflects the initial formation of a fusion pore. Exocytosis in PDEC was stimulated by increased [Ca2+]i and activation of PKA or PKC.
2. Membrane receptors on PDEC
- VIP, secretin, and prostaglandin receptors (8, 18).
VIP (0.1 µM) and both prostaglandins E1 and E2 (1 µM) stimulated increases in 123I- efflux from PDEC, suggesting that functional receptors for VIP and prostaglandins are expressed on these cells and can mediate the subsequent cAMP-mediated activation of CFTR (8). When PDEC were cultured with a feeder layer of normal human dermal myofibroblasts, secretin and VIP (both at 1 µM) stimulated increases in cAMP in early passages (3-5) but not in later passages (25-30), suggesting variable expression of these receptors by PDEC under different culture conditions and growth stages (18). - Histamine and angiotensin (AT) receptors (1, 13).
Histamine (10-100 µM) stimulated a 125I- efflux through Ca2+-activated Cl- channels (inhibited by NPPB, DPC, and DIDS) and a 86Rb+ efflux through Ca2+-activated K+ channels (inhibited by charybdotoxin). This effect was mediated by H1 receptors on the BLM of PDEC since it was i) inhibited by diphenhydramine, an H1 receptor antagonist, ii) resistant to cimetidine, an H2 receptor antagonist, iii) not reproduced by dimaprit, an H2 agonist, iv) inhibited by BAPTA, a Ca2+ chelator, and v) only elicited by serosal histamine (13).AT receptors were demonstrated on dog PDEC by western blot, with a lower density for AT2 vs. AT1 receptors. In patch clamp studies, AT activated an 11 pS non-selective cation channel, a 4.6 pS Na+ channel, a 3 pS anion channel, and an 8 pS Cl- channel (CFTR). Both apical or basolateral AT activated these channels (1).
- Proteinase-activated receptor-2 (PAR-2) effects on PDEC (4, 12).
PAR-2 is a G-protein coupled receptor that is activated when trypsin cleaves the extracellular N-terminus to expose a tethered ligand (SLIGRL-NH2), which in turn activates the cleaved receptor. Trypsin and the activating peptide (AP) corresponding to the tethered ligand, stimulated the Ca2+-activated Cl- channels (125I- efflux inhibited by DIDS and NPPB) and K+ channels (86Rb+ efflux inhibited by charybdotoxin). In Ussing chambers (Fig. 3), trypsin and AP stimulated an Isc when added to the serosal, but not apical, surface of PDEC monolayers. Immunostaining confirmed expression of PAR-2 on the BLM of dog pancreatic ducts. Thus, trypsin cleaves and activates PAR-2 on the BLM of PDEC.In follow-up studies, using indo-1 photometry to measure [Ca2+]i, PKC kinase assays, and carbon fiber amperometry to measure vesicular exocytosis, activation of PAR-2 was shown to promote intracellular Ca2+ mobilization, Ca2+ influx across the store-operated Ca2+ channel, and activation of protein kinase C (a and d isoforms), stimulating exocytosis and mucin secretion.
- Purinergic P2Y2 and P2Y11 receptors (9, 11).
- P2Y2 receptors (P2Y2-R)
Activation of P2Y2-R by ATP, UTP, ATP-g-S (and, to a lesser extent, by ß-g-methylene ATP, but not by adenosine) stimulated the Ca2+-activated Cl- channels (125I- efflux inhibited by NPPB, DPC, and DIDS) and K+ channels (86Rb+ efflux inhibited by BaCl2 and charybdotoxin). When either the apical membrane or BLM of confluent PDEC monolayers were permeabilized by nystatin and studied in Ussing chambers (Fig. 3), the serosal or luminal addition of UTP activated apical Cl- and basolateral K+ conductances, suggesting the expression of functional P2Y2-R on both the apical membrane and BLM. ATP, ATP-g-S, and UTP all stimulated mucin secretion through P2Y2-R. Thus, ATP activates P2Y2-R on the apical and basolateral membranes of PDEC to stimulate mucin secretion and ion transport. - P2Y11 receptors (P2Y-R):
P2Y11-R are unique P2Y receptors that are coupled not only to Gq, but also to Gs, to stimulate adenylate cyclase and cause an increase in cAMP. RT-PCR identified the mRNA for P2Y11-R in PDEC. Consistent with this finding, ATP, 2-methyl-S-ATP, and ATP-g-S, also activated P2Y11-R on PDEC to stimulate a 4-10 fold cAMP increase and activate the CFTR Cl- channel (125I- efflux resistant to BAPTA/AM). In Ussing chambers, only serosal ATP stimulated the cAMP-activated CFTR, localizing these receptors to the BLM of PDEC.
- P2Y2 receptors (P2Y2-R)
3. Integrated effects of ATP and bile acids on PDEC secretion
- Effects of ATP (2, 3).
- Effect on exocytosis:
Using Ca2+ photometry and amperometry, we observed that low concentrations of ATP (≤10 µM) induced [Ca2+]i oscillations but no significant exocytosis. In contrast, 100 µM ATP induced a sustained [Ca2+]i rise and a 7-fold increase in exocytosis, that was partially inhibited either by the Ca2+ chelator, BAPTA, or by the PKA inhibitors, H-89 or Rp-8-Br-cAMPS, and was further completely inhibited by the combined action of these agents. This finding suggests that ATP stimulated exocytosis through P2Y2-R (via increased [Ca2+]i), and through P2Y11-R (via increased cAMP). - Effect on K+ channels:Using UTP to activate the P2Y2-R, increasing [Ca2+]i (monitored through Ca2+ photomtery) and stimulating K+ currents (monitored by patch clamp studies) and/or exocytosis (monitored by amperometry), we observed that i) [Ca2+]i oscillations evoked by low UTP concentrations (1-10 µM) stimulated K+ current but not exocytosis, while the [Ca2+]i increases evoked by high UTP (100 µM) stimulated both K+ current and exocytosis, ii) [Ca2+]i oscillations induced more K+ current than a sustained [Ca2+]i rise, iii) K+ currents activated by UTP hyperpolarize the membrane, and iv) the corresponding K+ channels were sensitive to charybdotoxin, but not TEA, were of intermediate conductance (~50 pS), and showed a K1/2 of ~0.5 mM. Thus, UTP, acting via P2Y2-R, stimulated, at low concentrations, [Ca2+]i oscillations and K+ channels, and, at high concentrations, a sustained [Ca2+]i increase and exocytosis.
- Effect on HCO3- secretion:
CaM kinase II also mediated HCO3- secretion in dog PDEC. The activity of the Cl-/HCO3- exchanger was assessed as the [pH]i change induced by low extracellular Cl- (to drive paired Cl- efflux and HCO3- influx), measured with the pH-sensitive dye, BCECF-AM. Low Cl- stimulated an initial transient increase in [pH]i followed by a late sustained increase. ATP, at low concentrations (2-10 µM), only augmented the early transient increase but, at high concentrations (100 µM), augmented both phases. This effect was inhibited when i) DIDS inhibited the Cl-/HCO3- exchanger, ii) BAPTA-AM chelated intracellular Ca2+, and iii) KN-93 blocked CaM kinase II. Thus, ATP induced a [Ca2+]i increase to activate CaM kinase II and enhance the Cl-/ HCO3- exchanger activity.
- Effect on exocytosis:
- Effect of bile acids on secretion by PDEC (17).
Taurodeoxycholic acid (TDCA) stimulated a 125I- efflux inhibited by DIDS and NPPB and a 86Rb+ efflux inhibited by charybdotoxin. Inhibition by BAPTA suggests mediation by Ca2+ while the lack of LDH release excludes cellular toxicity. TDCA was more effective than its glycine-conjugated counterpart; taurochenodeoxycholate, another di-hydroxy bile acid, was similarly effective, but not taurocholate, a tri-hydroxy bile acid. In Ussing chambers, 1 mM serosal or 2 mM luminal TDCA stimulated an Isc increase from i) transepithelial transport, ii) apical Ca2+-activated Cl- channels, and iii) basolateral Ca2+-activated K+ channels.

Figure 3: Ussing chamber studies. Panel A: The Vitrogen-coated Snapwell insert supporting the cultured PDEC is mounted into an Ussing chamber so that the confluent monolayer forms a physical and electrical barrier between the serosal compartment, in contact with the bottom of the insert and the basolateral membrane of the PDEC, and the luminal compartment, in contact with the top of the insert and the apical membrane of the PDEC. Both sides of the monolayers are bathed with (in mM) 135 NaCl, 1.2 CaCl2, 1.2 MgCl2, 0.6 mM KH2PO4, 2.4 mM K2HPO4, 10 mM HEPES, and 10 glucose, warmed to 37oC with a circulating water jacket, and gently mixed and oxygenated with a constant inflow of 95% air/ 5% CO2. For secretory studies, the spontaneous tissue potential difference is short-circuited using a voltage clamp with Ag-AgCl2 electrodes, and the current necessary to maintain this short circuit (Isc), which counteracts and reflects the electrogenic flow of ions across the tissue from one compartment to the other, is recorded. To determine the transepithelial resistance, a current of 100 µA is maintained across the monolayer, the resulting voltage recorded, and the resistance calculated using Ohm’s law: voltage = current x resistance. Our Ussing chamber, from Physiologic Instruments, San Diego, CA, includes: voltage clamp (VCCMC2), chambers (P2300), sample holder (P2302), electrode set (P2020-S), and data acquisition software (Acquire & Analyze rev II).
Panel B: Under regular conditions, the Isc reflects the net electrogenic flow of ions across the epithelial cell, mediated through different ion transport pathways on the basolateral and apical membranes of the PDEC (top cell). When one side of the cell is permeabilized with 0.36 mg/ml nystatin, the intracellular ion concentration will equilibrate with the adjacent compartment, allowing ion transport pathways on the opposite membrane to be studied (e.g. middle cell shows basolateral membrane permeabilization, equilibration between the serosal and intracellular compartments, and potential studies of apical ion channels). Specific ion depletion will provide the driving chemical gradient to study the corresponding ion transport pathway (e.g. in the bottom cell, a Cl- gradient generated by depleting chloride in the luminal compartment will allow studies of Cl- ion transport across the apical membrane).
D. Future Opportunities with Dog PDEC Cultures
While the current dog PDEC culture system has allowed us to make inroads into understanding the (patho)physiology of pancreatic ductal secretion, additional developments will improve this model. These opportunities include culture of PDEC from intralobular pancreatic ducts, as these cells may display more robust bicarbonate secretion, extension of these techniques to PDEC from other species, culture and monitoring systems that mimic the small tubular structure of pancreatic ducts, and, as always, simpler culture methods. Hopefully future advances will mirror past progress.
E. References
- Fink AS, Wang Y, Mendez T, Worrell RT, Eaton D, Nguyen TD, and Lee SP. Angiotensin II evokes calcium-mediated signaling events in isolated dog pancreatic epithelial cells. Pancreas 25: 290-295, 2002. PMID: 12370541
- Jung SR, Kim K, Hille B, Nguyen TD, and Koh DS. Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. J Physiol 576: 163-178, 2006. PMID: 16857709
- Jung SR, Kim MH, Hille B, Nguyen TD, and Koh DS. Regulation of exocytosis by purinergic receptors in pancreatic duct epithelial cells. Am J Physiol Cell Physiol 286: C573-579, 2004. PMID: 14602582
- Kim MH, Choi BH, Jung SR, Sernka TJ, Kim S, Kim KT, Hille B, Nguyen TD, and Koh DS. Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. J Biol Chem 283: 18711-18720, 2008. PMID: 18448425
- Koh DS, Moody MW, Nguyen TD, and Hille B. Regulation of exocytosis by protein kinases and Ca(2+) in pancreatic duct epithelial cells. J Gen Physiol 116: 507-520, 2000. PMID: 11004201
- Kuver R, Savard C, Nguyen TD, Osborne WR, and Lee SP. Isolation and long-term culture of gallbladder epithelial cells from wild-type and CF mice. In Vitro Cell Dev Biol Anim 33: 104-109, 1997. PMID: 9081217
- Logsdon CD. Pancreatic duct cell cultures: There is more to ducts than salty water. Gastroenterology 109: 1005-1009, 1995. PMID: 7657086
- Nguyen TD, Koh D-S, Moody MW, Fox NR, Savard CE, Kuver R, Hille B, and Lee SP. Characterization of two distinct chloride channels in cultured dog pancreatic duct epithelial cells. Am J Physiol 272: G172-180, 1997. PMID: 9038891
- Nguyen TD, Meichle S, Kim U-S, Wong T, and Moody MW. P2Y11, a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol 280: G795-G804, 2001. PMID: 11292586
- Nguyen TD, and Moody MW. Calcium-activated potassium conductances on cultured non-transformed dog pancreatic duct epithelial cells. Pancreas 17: 348-358, 1998. PMID: 9821176
- Nguyen TD, Moody MW, Savard CE, and Lee SP. Secretory effects of ATP on non-transformed dog pancreatic duct epithelial cells. Am J Physiol 275: G104-113, 1998. PMID: 9655690
- Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh D-S, and Bunnett NW. Trypsin activates pancratic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest 103: 261-269, 1999. PMID: 9916138
- Nguyen TD, Okolo C, and Moody MW. Histamine stimulates secretion by non-transformed pancreatic duct epithelial cells. Am J Physiol 275: G76-84, 1998. PMID: 9655687
- Oda D, Lee SP, and Hayashi A. Long-term culture and partial characterization of dog gallbladder epithelial cells. Lab Invest 64: 682-692, 1991. PMID: 1709426
- Oda D, Savard CE, Eng L, and Lee SP. The effect of N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) on cultured dog pancreatic duct epithelial cells. Pancreas 12: 109-116, 1996. PMID: 8720655
- Oda D, Savard CE, Nguyen TD, Eng L, Swenson ER, and Lee SP. Dog pancreatic duct epithelial cells: long-term culture and characterization. Am J Pathol 148: 977-985, 1996. PMID: 8774152
- Okolo C, Wong T, Moody MW, and Nguyen TD. Effects of bile acids on dog pancreatic duct epithelial cell secretion and monolayer resistance. Am J Physiol Gastrointest Liver Physiol 283: G1042-1050, 2002. PMID: 12381517
- Zhang M, Schleicher RL, Fink AS, Gunter-Smith P, Savard C, Nguyen T, and Lee SP. Growth and function of isolated canine pancreatic ductal cells. Pancreas 20: 67-76, 2000. PMID: 10630386


