Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.27
| Attachment | Size |
|---|---|
| 537.77 KB |
Introduction
Pancreatitis is a known complication of chronic alcohol abuse with both acute (necroinflammation) and chronic (fibrosis and acinar atrophy) manifestations. The necrosis-fibrosis sequence, wherein alcoholic chronic pancreatitis evolves from repeated episodes of acute injury eventually leading to irreversible damage and loss of function, is now widely accepted. This concept is supported by the clinical observation that patients with frequent attacks of alcoholic pancreatitis progress more rapidly to chronic disease. However, while it is acknowledged that the risk of alcoholic pancreatitis increases with increasing consumption of alcohol, less than 5% of heavy drinkers develop clinically overt pancreatitis, suggesting that an additional trigger factor is required to initiate overt pancreatic injury (1, 11). Lipopolysaccharide (LPS or endotoxin, derived from the cell wall of gram negative bacteria) is a potential, clinically significant trigger factor since elevated blood endotoxin concentrations (derived from gram negative bacteria in the gut) are found in alcoholics, even after a single binge (5). In addition, blood endotoxin levels have been shown to correlate with the severity of pancreatitis (2).
It is noteworthy that long term ad libitum administration of alcohol in rats does not cause any pancreatic injury thus concurring with the human situation (8). Hence, additional stimuli (such as caerulein administration, caerulein and cyclosporin, trinitrobenzene sulfonic acid (TNBS) etc.) have been applied to induce acute and chronic lesions in the pancreas. While these models have contributed to the understanding of alcoholic pancreatitis, none of these factors is of proven physiological relevance.
Building on an early model of alcoholic hepatitis by Bhagwandeen and Apte et al (3) and a more recently developed model of alcoholic acute pancreatitis (6), we have established a rat model of alcoholic pancreatitis based on ethanol administration (Lieber-DeCarli liquid diets) and repeated intravenous injections of LPS from E.Coli. This model recapitulates the key features of human chronic pancreatitis, i.e. acinar atrophy and fibrosis (9). More importantly, pancreatic lesions regress completely within a week after alcohol withdrawal (10).
This chapter will present the methodology for the preparation of alcohol diets, group feeding and LPS administration in rats.
1.0 Material
- Liquid diet feeding tubes (100ml), Bio-Serv, Frenchtown, NJ 08825
- Liquid diet feeding tube holders, Bio-Serv, Frenchtown, NJ 08825
- Wire-bottom cages (custom-made)
- Broome style rodent restrainer, n° 554-BSRR (rats), Plas-Labs, Lansing, Michigan 48906
- Broome style rodent restrainer, n° 551-BSRR (mice), Plas-Labs, Lansing, Michigan 48906
1.1 Reagents
1. Preparation of Lieber-DeCarli liquid diets
Introduction:
Work by Charles Lieber has greatly shaped our understanding of alcoholic liver disease. One of Lieber’s major achievements was to prove that alcohol consumption per se (and not dietary deficiencies) led to liver damage. In 1963 Charles Lieber and Leonore DeCarli established ethanol feeding as part of a liquid diet (7). In this diet ethanol, when present, is substituted for carbohydrates and comprises 36% of the total calories. The alcohol diet and the control diet contain the same amount of calories per volume of diet. This facilitates the administration of isocaloric amounts (=similar amounts of calories, i.e. similar volumes) of diet to study animals and their respective controls (pair feeding or group feeding depending on the number of controls).
Table 1. Nutrient content of Lieber-DeCarli liquid diets

Unless stated otherwise, all chemicals are of analytical reagent grade and available from the Sigma Chemical Company (St Louis, Missouri, USA).
a) Vitamin mixture

b) Salt solution
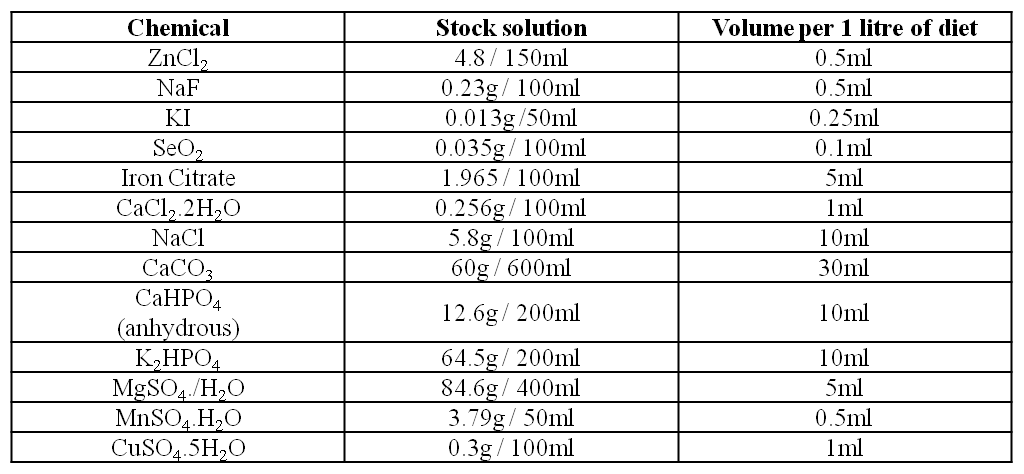
Note: Stock solutions are prepared the week before commencing the diet and are stable at room temperature for the duration of the feeding period.
c) Oil Mixture
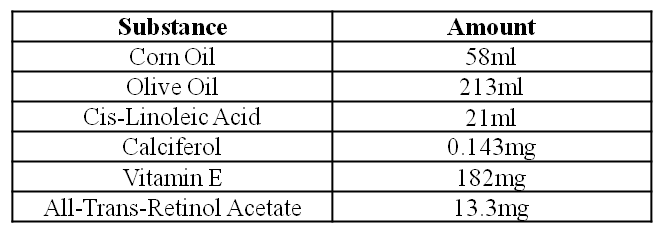
Note: . The mixture is stirred at room temperature until no precipitate can be seen. This takes approximately 1 hour. The oil mixture is stable for 3 days at 4°C in the dark
d) Control Liquid Diet
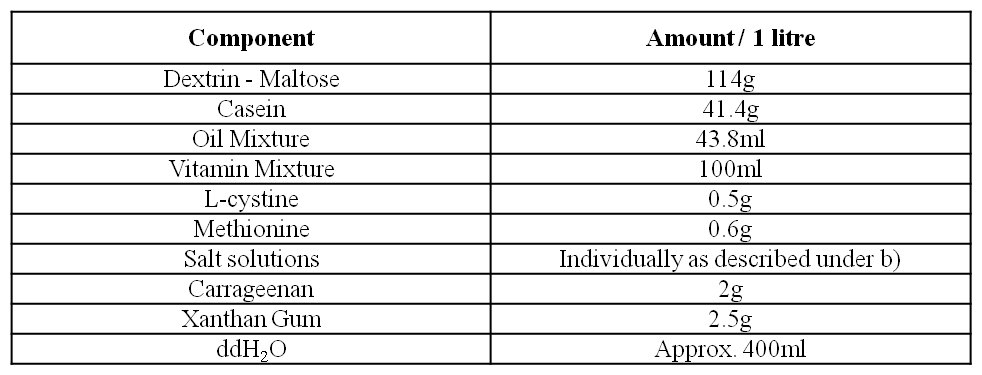
The different components of the diet are added into a blender according to the Table above. Both carrageenan and xantham gum are sprinkled evenly over the ingredients in order to avoid the formation of clumps in the diet. The diet is then mixed in the blender (3 – 4 pulses of 10 seconds each). The diet subsequently made up to the final volume with double deionised H2O. The control diet is stored for a maximum of 3 days at 4°C.
e) Alcohol Liquid Diet
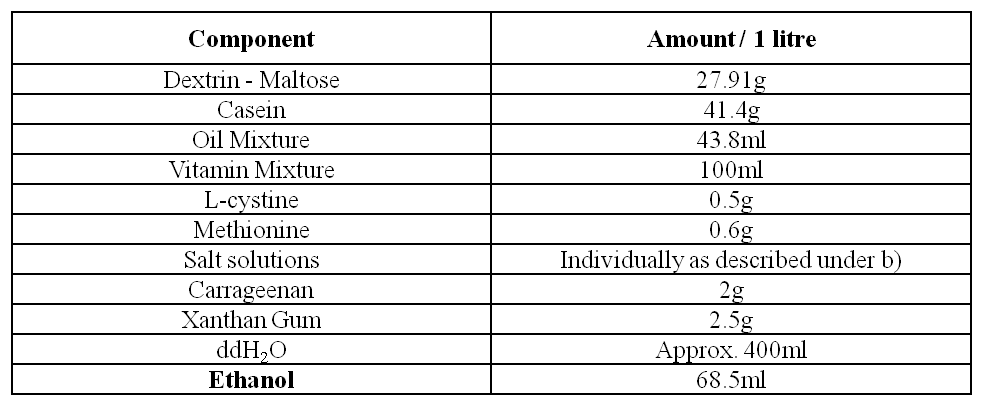
The different components of the diet are added into a blender according to the Table above. Alcohol is added last. The diet is then mixed by a blender as described above. The diet is made up to the desired volume with ddH2O. The alcohol diet is stored for a maximum of 3 days at 4°C.
2. Preparation of LPS solution
- LPS from E.Coli B26:026 (1mg/vial), lyophilised powder, n° L2654, Sigma Chemical Company (St Louis, Missouri, USA.
- NaCl 0.9% (sterile), Becton Dickinson (BD)
LPS from the E.Coli strain B26:026 is diluted in NaCl 0.9% to a final concentration of 5 mg/ml (200ml of NaCl 0.9% per vial) immediately before use. The solution should be at room temperature by the time of injection.
2.0. Methods
1. Administration of Lieber-DeCarli liquid diets
Littermate, male, weanling Sprague-Dawley rats (110-130g) are individually housed in wire-bottom cages (Figure 5) and fed with isocaloric amounts (i.e. identical volumes) of Lieber-DeCarli liquid diets. Since the two variables in this model are alcohol and LPS the following groups are, in principle, required :
i) Control diet fed rats (C);
ii) Alcohol-fed rats (A);
iii) Control-diet fed rats receiving LPS injections (CL);
iv) Alcohol-fed rats receiving LPS injections (AL).
Diets are adjusted within a quartet of animals, comprising one representative of each group. It is recommended to include animals of comparable weight (+/- 5%) into a given quartet as their caloric requirements are likely to be similar.
The dietary intake of each animal in the quartet is matched so that all rats in the quartet receive isocaloric amounts of the diet. The rate limiting animal (the rat whose daily intake determines the daily intake of the other 3 animals) is usually an alcohol-fed rat. The amount of diet to be administered is calculated and adjusted on a daily base. It is recommended to feed the animals at the same time of the day (usually in the morning).
Alcohol diet is introduced progressively using the following scheme:
Feeding is pursued for 10 weeks prior to LPS injection. Individual diet consumption is recorded in a log book.
2. LPS tail vein injections
This procedure is performed in a dedicated quiet area outside of the animal feeding room (Figure 1). It is recommended to take one animal at the time within its cage. The cage should be wrapped into a towel. Rats are retrieved from the cages, restrained in an animal restrainer and weighed. The rat tail is warmed up using an infrared light or warm water. The site of injection is chosen as distally as possible and disinfected with ethanol. The volume of LPS solution to be injected is calculated according to the bodyweight (3mg/kg). This procedure is repeated twice in weekly intervals (total of 3 injections). Alcohol / control diet administration continues throughout the injection period.

Figure 1: Preparation for tail vein injections. It is recommended to prepare a cone shaped fabric with a small (5mm wide) hole at its tip. The rat seeks shelter in the fabric (A). The animal is inserted into the restrainer (B). The rat tail is warmed up in a water bath (C). Injection of LPS/saline solution into the distal portion of the vein (D).
3. Sacrifice of experimental animals and organ processing
Animals are sacrificed 24 hours after the last LPS / saline injection by decapitation. Pancreata are retrieved and cut into 3 parts (proximal, middle and distal pancreas). This is in order to avoid sampling error since lesions may patchy. Samples of each region are divided into pieces and may be processed as follows: i) snap freezing in liquid nitrogen, ii) embedding in OCT compound medium (for frozen sections) and iii) fixation in 10% neutral buffered formalin.
Acute lesions (acinar cell necrosis, vacuolisation, inflammatory infiltrate, haemorrhage) are best evaluated on hematoxylin&eosin stained sections.
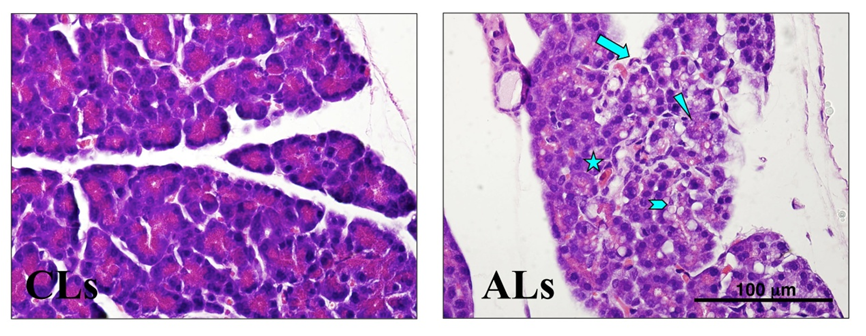
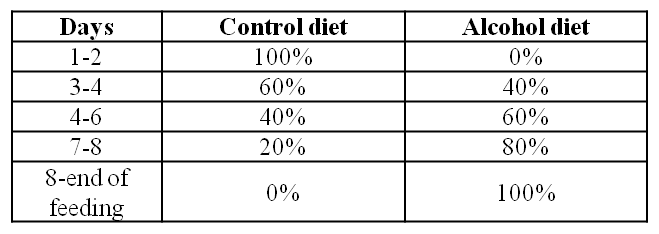
Figure 2: Effect of ethanol ± single LPS injection on the rat pancreas. Representative H&E stained pancreatic sections from rats fed a control diet receiving a single LPS injection (CLs) and rats fed an alcohol diet receiving a single LPS injection (ALs). ALs rats displayed significant acinar vacuolisation (block arrow), acinar cell necrosis (arrowhead), inflammatory infiltrate (arrow) and haemorrhage (star). Negligible lesions were found in the CLs group. Original magnification x600.
Fibrosis is assessed on Masson’s Trichrome (Figure 3) or Sirius Red stained sections. The extent of collagen deposition can be further assessed by the hydroxyproline assay.
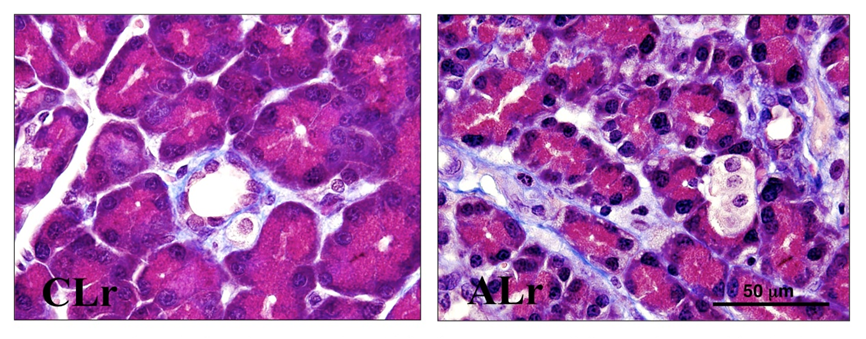
Figure 3: Effect of ethanol ± repeated LPS injections on extracellular matrix deposition in the rat pancreas. Representative Masson’s trichrome stained pancreatic sections from rats fed a control diet receiving repeated LPS injections (CLr) and rats fed an alcohol diet receiving repeated LPS injections (ALr). Pancreata of ALr rats displayed significant periacinar fibrosis (blue stained bands) and acinar atrophy. In CLr animals, blue staining was confined to periductular and perivascular regions and acinar size was normal. Original magnification x1000.
Immunostaining for α smooth muscle actin (Figure 4) confirms activation of pancreatic stellate cells in alcohol-fed animals receiving repeated LPS injections.
Figure 4: Effect of ethanol ± repeated LPS injections on alpha smooth muscle actin (αSMA) expression in the rat pancreas. Representative paraffin-embedded sections immunostained for αSMA from rats fed a control diet receiving repeated LPS injections (CLr) and rats fed an alcohol diet receiving repeated LPS injections (ALr). Pancreata of ALr rats displayed marked periacinar immunoreactivity for αSMA indicating the presence of activated pancreatic stellate cells. Original magnification x600. Antibody: Monoclonal mouse anti- α -SMA antibody, n° A5228 – clone 1A4, Sigma, St. Louis, Missouri, USA.
3.0 Notes
1. Animals from the feeding model should be housed in a dedicated, well ventilated room, isolated from other experimental animals. Alcohol-fed rats are extremely sensitive to brisk noises, particularly after the injections. It is recommended to have the radio on at all times (low volume) and to avoid unnecessary visits to the animal room. Animals are to be handled very carefully. Alcohol rats in particular are irritable and easily stressed. This may lead to inappetence, alcohol withdrawal symptoms and death.
2. Wire bottom cages are used to prevent the ingestion of bedding or feces by the animals (Figure 5). Animal ethic committees may recommend some environmental enrichment (such as a tunnel). Any environmental enrichement should be made of metal.
3. Animals fed Lieber and DeCarli liquid diets do not need any additional water .The water provided in the diet is sufficient for their daily requirements. However, animal ethic committees may ask for a separate water supply.
4. According to the literature, Lieber-DeCarli liquid diets have been successfully used with other rat strains (e.g. Wistar rats). However, the rat model described above has, to the best of our knowledge, been implemented only with Sprague-Dawley rats.
5. We advice against the use of permanent intravenous lines for LPS administration in rats. The latter represent a source of irritation and a potential cause of infection for the animals.
6. The model described above has been applied C57BL6 male mice (12). Our preliminary studies had shown that this mouse strain did not tolerate ethanol concentrations of 5% v/v. This may be due to the fact that C57BL6 male mice are heavier drinkers than Sprague-Dawley rats and/or that they are particularly sensitive to the effects of ethanol. Hence, the recommended ethanol concentration of the alcohol diet is 3% v/v. Alcohol at this concentration in conjunction with intravenous LPS reproduces the acute and chronic pancreatic lesions observed in Sprague-Dawley rats (12; Figure 5).
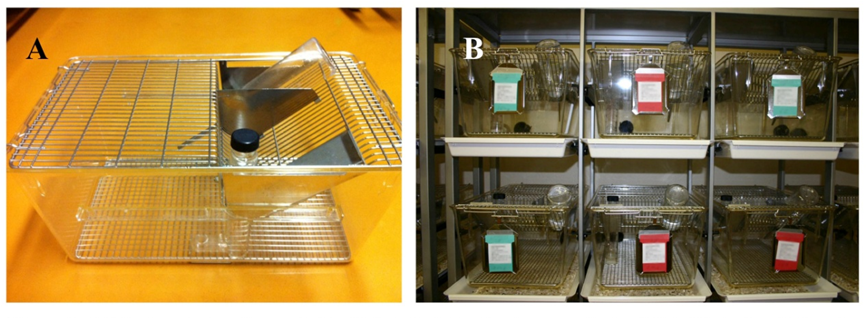
Figure 5: (A) Custom-designed cage for liquid diet administration in rats and mice (Alain Vonlaufen, University of Geneva, Switzerland; Indulab®, CH-9473 Gams). Feeding tubes and water bottles are inserted from top of the cage. (B) Individual housing of mice fed Lieber-DeCarli liquid diets. Mice rest on a 7x7mm grid (rats: 11x11mm). Bedding is placed in a removable plate underneath the cage.
4.0 References
- Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology 111: 224-31, 1996. PMID: 8698203
- Ammori BJ, Leeder PC, King FR, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure and mortality. J Gastrointest Surg 3: 252-62, 1999. PMID: 10481118
- Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol 25: 1816-26, 2010. PMID: 21091991
- Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol 152: 47-53, 1987. PMID: 3305847
- Bode C, Kugler V, Bode JC. Endotoxiemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 4: 8-14, 1987. PMID: 3571935
- Fortunato F, Deng X, Gates LK, McClain CJ, Bimmler D, Graf R, Whitcomb DC. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis? Am J Physiol Gastrointest Liver Physiol 290: G232-41, 2006. PMID: 15976389
- Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update
- Alcohol Alcohol 24: 197-211, 1989. PMID: 2667528
- Singh M, LaSure MM, Bockman DE. Pancreatic acinar cell function and morphology in rats chronically fed an ethanol diet. Gastroenterology 82: 425-34. PMID: 6172313
- Vonlaufen A, Xu Z, Daniel B, Kumar RK, Pirola R, Wilson J, Apte MV. Bacterial endotoxin: A trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 133: 1293-303, 2007. PMID: 17919500
- Vonlaufen A, Phillips PA, Xu Z, Zhang X, Yang L, Pirola RC, Wilson JS, Apte MV. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 60(2): 238-46, 2011. PMID: 20870739
- Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lawrence C, Elinoff B, Greer JB, O'Connell M, Barmada MM, Slivka A, Whitcomb DC; North American Pancreatic Study Group. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 169: 1035-45, 2009. PMID: 19506173
- Vonlaufen A, 2009, “Alcohol, Endotoxin and the Pancreas: Progression and Reversibility of Alcoholic Pancreatitis”, PhD thesis, The University of New South Wales, Sydney, NSW, Australia.


