Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.21
| Attachment | Size |
|---|---|
| 245.74 KB |
Introduction
Early measurements of cholecystokinin (CCK) were based on the abilities of blood or blood extracts to stimulate gallbladder contraction or pancreatic exocrine secretion. These biological assays were performed in laboratory animals such as dogs, pigs or rodents. The usefulness of these assays was limited because methods were cumbersome and interpretation of results was complicated by confounding problems introduced by other hormones or neural influences. Invention of radioimmunoassay revolutionized the study of endocrinology and most hormones can now be measured by this method. However, the radioimmunoassay of CCK was particularly difficult for several reasons (9). First, multiple molecular forms of CCK ranging from CCK-4 to CCK-83 have been found in blood. Although it appears as though CCK-58 is the major circulating form in the species examined, other forms may contribute to CCK activity. Second, CCK is structurally similar to gastrin. Both CCK and gastrin have an identical pentapeptide carboxyl terminus. Therefore, to avoid cross-reactivity, assays must be able to detect the regions of CCK that are distinct from gastrin. Third, CCK circulates in the blood at concentrations that are much lower (10-100 fold) than gastrin. Fourth, large molecular forms of CCK (e.g., CCK-58) that can be used as standards in assays are not routinely available. Fifth, isotope labeling of CCK can be difficult. Sixth, CCK has posttranslationally modified amino acids (e.g., sulfated tyrosine) that are important for activity. CCK-8 is fully active and more potent than shorter forms of CCK. Full activity requires that (1) the hormone be sulfated in position seven (from the carboxyl terminus) and (2) methionine at position 3 remain in the reduced (non-oxidized) state. This multitude of potential problems delayed the development of reproducible, sensitive, and specific RIAs for CCK.
Reliable CCK assays must be capable of detecting low levels of CCK yet distinguish CCK from gastrin (Figure 1). This means that for radioimmunoassays, antibodies must recognize the three amino acids that are common to all forms of CCK (CCK-8 and larger) but are not found in gastrin. To measure fully biologically active CCK, antibodies must also distinguish sulfated from non-sulfated CCK. Finally, preparation of radiolabeled CCK for RIA must avoid harsh conditions that oxidize the molecule. To circumvent the obstacles posed for establishing a CCK RIA, a sensitive and specific CCK bioassay was developed using an in vitro preparation of pancreatic acini. This method was made possible because of the sensitivity of the CCK1 receptor expressed on rodent pancreas and its selectivity for CCK over gastrin.

Figure 1. The amino acid sequences of human gastrin-17 and CCK-8. Both gastrin and CCK are linear peptides. Note that the carboxyl terminus (right hand side) of each peptide is amidated. Both peptides share an identical carboxyl terminal pentapeptide sequence (shown in green). However, the three amino acids at the amino terminus of CCK-8 are unique (red) and, importantly, contain a sulphated tyrosine residue. Therefore, antiserum that can detect CCK-8 without cross-reactivity with gastrin must recognize this region.
There are also several good CCK RIAs reported in the literature, one of which is commercially available. I will review our methods for using both bioassay and RIA and then describe considerations in choosing the most appropriate assay.
Bioassay of CCK
The bioassay of CCK is based on the ability of CCK to stimulate amylase release from isolated rat pancreatic acini (10, 11). It has been used to measure plasma CCK in rats, mice, and humans (10, 11, 14) but could be extended to other species. At the core of this assay is the technique for preparing viable acini that retain their sensitivity to very low concentrations of CCK. The method for isolation of acini has been very well described by John Williams in the Pancreapedia (17) and we will refer to his method for many details.
Collection of blood and extraction of CCK from plasma
Materials
- Sep-Pak Classic C18 Cartridges, 360 mg, 55-105μm (Waters WAT051910) from Waters Associates
- Distilled Water
- Methanol (HPLC grade) – We have found that methanol must be from bottles. Methanol in aluminum containers does not work.
- Syringes (3-10 mL) or vacuum apparatus
- Ethanol
- Trifluoroacetic acid (1%) (Sigma-Aldrich, catalog # T6508)
- Blood dilution vials (flat bottomed) (VWR, catalog #14310-684)
Method for collection of plasma
- We typically collect blood from rats and mice by cardiac puncture. However, we have also collected trunk blood following decapitation and found similar plasma CCK levels. In humans, blood is typically collected by venipuncture. Immediately following collection, blood is placed into tubes containing ~100 U sodium heparin and placed on ice.
- Blood is centrifuged at 4°C to separate plasma.
- Within 30 minutes of blood collection, plasma must be passed through activated Sep-Pak cartridges.
CCK Extraction onto Sep-Pak Cartridges
- Attach 3-10 mL syringe to Sep-Pak cartridge. This can be done with either a syringe plunger or vacuum for running cartridges as columns. Cartridges should be oriented so loading and elution of liquid is always in the same direction. Loading of cartridges is performed at room temperature. Cartridges should be marked in such a way to ensure adequate identification. We have found that solvents can remove pen markings so it is often useful to cover identifiers with cellophane tape.
- Activate Sep-Pak cartridge with 5 mL methanol that is passed through the cartridge.
- Wash each cartridge with 20 mL distilled water.
- Load the plasma (1-6 mL) onto the cartridge slowly at a rate of ~ 2 mL/minute. The amount of sample is generally a function of the species used. For basal plasma CCK several mL are necessary and for mice this may require pooling samples.
- Record volume of plasma applied to each Sep-Pak.
- Rinse each cartridge with 20 mL distilled water
- Dry Sep-Pak by squirting air from an empty syringe or opening vacuum to air to blow out any liquid
- Sep-Pak cartridges can then be wrapped in plastic wrap and stored at -80oC until assay.
Elution of CCK from Sep-Paks
- On the day of assay, Sep-Pak cartridges are removed from the freezer and thawed at room temperature.
- Make fresh Elution buffer which is comprised of 4 parts ethanol, 1 part 1% TFA.
- Each cartridge is washed with 20 mL distilled water
- Using a syringe attached to the cartridge, CCK is eluted from the Sep-Pak into a flat-bottomed blood dilution vial with 1 mL Elution buffer. All of the liquid is eluted from the Sep-Pak cartridge by squirting air from an empty syringe into the cartridge.
- Flat-bottomed vials are placed in a water bath at 45oC and evaporated to dryness under a continuous nitrogen stream
- When dry (it generally takes 30-45 minutes) vials are ready for use in the bioassay (see Measurement of Amylase Secretion Dose Response Curve).
Isolation of pancreatic acini
Materials
Materials for isolation of pancreatic acini for bioassay of plasma CCK are those described in “Isolation of Rodent Pancreatic Acinar Cells and Acini by Collagenase Digestion” by Williams (17) with the exception that we use a Tris Ringer for suspending acini immediately prior to incubation with CCK and plasma extracts. Tris is used in place of KHB or HEPES, the buffer previously used in studies of isolated pancreatic acini, as it provides greater buffering capacity and gives more consistent results when incubated with plasma extracted with ethanol and trifluoroacetic acid. Tris Ringer is oxygenated with 100% O2, instead of 5%CO2/95%O2.
Tris Ringer (100 mL) contains:
- 40 mM Tris (hydroxymethyl) aminomethane
- 103 mM NaCl
- 1 mM NaH2PO4
- 4.7 mM KCl
- 1.28 mM CaCl2
- 0.56 mM MgCl2
- 11.1 mM glucose
- 0.1 mg/mL soybean trypsin inhibitor
- 2 mL minimal Eagle’s medium amino acid supplement (GIBCO, catalog #211130)
- 5 mg/mL bovine serum albumin (universal grade BSA) (Millipore, catalog # 81-003-3)
- Adjusted to pH 7.58 at room temperature
Methods
The method we use for preparing isolated pancreatic acini was adapted from that described by Williams (17). Referring to the description in the Pancreapedia, our methods are identical for section 2.1 steps 1-8. At step 9 we substitute Tris Ringer for the Incubation solution (referred to as solution I). Tris Ringer is oxygenated with 100% O2 and is used with acini throughout the remainder of the protocol.
- Prepare experimental solutions substituting Tris Ringer for solution “I”. Bovine serum albumin (100 mg) is dissolved in 100 mL Tris Ringer by pouring it on top of the solution and letting it dissolve without shaking or stirring. The solution is kept under a slow flow of O2 until used.
- Pancreas is collected from one young adult male rat (150-180 gm). In our original report (11), we used female rats and found that ovariectomy reduced the variability in CCK responsiveness. However, we subsequently discovered that pancreas from young rats was more responsive than that from older rats and pancreas from young male rats was very similar to that of ovariectomized females.
- Inject pancreas with collagenase solution and initiate digestion.
- Change buffer and continue collagenase digestion of pancreas.
- Disperse pancreatic acini and filter pancreatic acini through nylon mesh.
- Centrifuge acini through BSA gradient.
- Transfer acini to Tris Ringer. Remove supernate and resuspend pellet in 5 mL of Tris Ringer, combine into one tube and let acini settle out or centrifuge.
- Pre-incubate acini in TR after gassing with O2.
- Centrifuge, collect acini and disperse in 25-75 mL Tris Ringer.
Measurement of Amylase Secretion Dose Response Curve
At this point plasma extracts in blood dilution vials that have undergone drying under nitrogen are inserted into the protocol in section 2.2 “Measurement of Amylase Secretion Dose Response Curve.” In step 4, 1 mL aliquots of acini suspended in Tris Ringer are placed into blood dilution vials to which standard concentrations of CCK-8 are added. Acini (1 mL) but no CCK-8 are added to vials containing plasma extracts. After addition of acini with or without CCK-8, all vials are handled in a similar manner as described in steps 5-7.
- Dilute CCK-8 for a dose-response curve (0-1,000 pM).
- Label blood dilution vials.
- Incubate individual aliquots of acini with standard concentrations of CCK or plasma extracts. Place 1 mL aliquots of acini into microcentrifuge tubes ToA and incubation vials 1 and 2. Immediately cap and centrifuge the ToA sample for 20 seconds and place in an ice bucket. Add 10 μL of concentrated secretagogue to vials 1 and 2, gas, cap, and place in 37°C water bath shaking at 50-60 cycles per minute. Start timer at zero and note time on procedure sheet. Repeat for the remaining samples to which CCK-8 is added. Aliquot 1 mL of acini into microcentrifuge tube ToB, centrifuge for 20 seconds and place in an ice bucket. Place 1 mL aliquot of acini into blood dilution vials containing dried plasma extracts, gas, and place in water bath noting the time of each entry. Repeat two at a time until all vials with plasma extracts have been placed into incubation. Place 1 mL of acini into microcentrifuge tube ToC, centrifuge for 20 seconds and place in an ice bucket. Place 1 mL aliquots of acini into two blood dilution vials, add 10 μL of concentrated secretagogue, gas, and place in water bath. Place 1 mL of acini into microcentrifuge tube ToD, centrifuge for 20 seconds and place in an ice bucket. Carefully remove the supernatant liquid from the To tubes and place in clean tubes.
- Remove samples from water bath and terminate incubation by centrifugation.
- Sonicate samples of acinar pellets.
- Assay amylase in each sample.
Calculation of plasma CCK concentration
At the conclusion of the bioassay, each sample will calculated as “per cent amylase release”. The amount of amylase is proportional to the amount of CCK present in plasma. A standard curve is constructed plotting amylase release as a per cent of total amylase content vs. CCK concentration (Figure 2). From this standard curve the concentration of CCK in each sample can be calculated. The actual plasma concentration is then determined by dividing the CCK value by the volume of plasma placed onto each Sep-Pak cartridge.
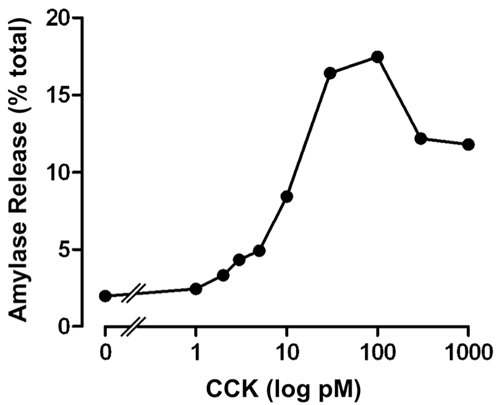
Figure 2. CCK dose-response for stimulation of amylase release from isolated pancreatic acini. CCK concentrations from each sample are determined from the “amylase release (% of total)” by dividing the CCK value by the original plasma volume. The graph shows data from a representative experiment. Each value in the standard curve is the average of duplicate samples.
CCK concentrations as low as 2 picomolar can generally be detected. By concentrating 2 – 6 mL of plasma in a Sep-Pak cartridge, it is possible to detect blood levels of 0.4 - 1 picomolar. The intra-assay and inter-assay variabilities are <20%.
We have performed a number of control experiments to confirm that bioactivity in rodent and human plasma extracts is due to CCK and not some other pancreatic secretagogue (10, 11). Normal blood levels of gastrin, secretin, and VIP do not interfere in this assay. However, it is conceivable that exogenously administered drugs that either block CCK receptor binding or otherwise inhibit acinar cell secretion would cause falsely low CCK measurements. Alternatively, drugs that stimulate or potentiate pancreatic acinar cell secretion could give falsely high CCK values.
Radioimmunoassay
One of the major problems that has plagued the CCK field has been difficulty in developing sensitive and specific CCK assays. Several radioimmunoassays and a bioassay have been developed that have sufficient sensitivity to detect what are low basal levels of CCK in plasma (1, 2, 5-8, 11, 13, 15). Another problem has been the appearance of various molecular forms of CCK in blood. Molecular forms ranging in size from CCK-8 to CCK-58 have been described in dogs, rats, and humans (3, 4, 16).
To circumvent these problems reliable RIAs must be sensitive enough to detect blood CCK levels in the low picomolar range and not cross-react with gastrin with which CCK shares an identical carboxy-terminal pentapeptide. Because multiple molecular forms of CCK may exist in plasma, an ideal CCK antisera would recognize all biologically active molecular forms. Since the carboxyl terminus is the biologically active end of CCK, antisera must recognize the three amino terminal amino acids that are unique to CCK-8 (figure 1). Several antisera are now available. One of the best characterized is that developed by Rehfeld (13). This antiserum (Ab. 92128), raised in rabbits, binds sulfated CCK-8, CCK-22, CCK-33, and CCK-58 with nearly equimolar potency and essentially no cross-reactivity with gastrin.
A commercially available CCK RIA kit known as EURIA-CCK provided by ALPCO Diagnostics, American Laboratory Products Company references Rehfeld (13) and uses an antiserum that appears to have reasonable selectivity for CCK over gastrin. Our laboratory has used this method and because it is readily available, its method is described below (12). Note that the method for collecting and extracting CCK from blood differs from the CCK bioassay.
Principle of the Method
CCK is extracted from plasma by an ethanol extraction method. CCK in extracts is assayed by a competitive radioimmunoassay using an antiserum raised in rabbits against sulphated CCK-8 N-terminally conjugated to bovine albumin. CCK in standards and samples compete with 125I-CCK-8 in binding to antibodies. Sulphated 125I-CCK-8 binds in a reverse proportion to the concentration of CCK in standards and samples. The assay is standardized against sulphated CCK-8. Antibody-bound 125I-CCK-8 is separated from the unbound fraction using double antibody solid phase. The radioactivity of the bound fraction is measured in a gamma counter.
Assay Procedure
The assay is performed in two stages: (1) extraction of plasma samples and (2) radioimmunoassay of extracts.
Extraction Procedure
Human blood is collected by venipuncture and placed into tubes containing EDTA. Plasma is separated by centrifugation and 1mL of each plasma sample kept on ice is mixed with 2 mL of chilled 96% ethanol. Each sample is vortexed and following a 10 minute incubation, centrifuged at 1700 x g for 15 minutes. The supernatant containing CCK is decanted and evaporated in a Speed Vac Concentrator at 37°C. Samples are stored at –20°C until the day of assay.
Alternative method for collection of blood
Vein blood is collected in tubes containing EDTA and Trasylol® (5000 KIU Trasylol in a 10 mL vacutainer). The sample is immediately cooled in an ice-bath. Plasma is separated by centrifugation using a refrigerated centrifuge. The plasma should be frozen within 2 hours and stored at -20°C or lower until assayed. Repeated thawing and freezing must be avoided.
For samples designated “accuracy”, exogenous CCK-8 (10 μL of a 10-9 M solution of sulphated CCK-8) is added to each 1 mL sample. This should yield a CCK measurement 10 pM greater than the corresponding basal value and is used as a control for sample handling and extraction conditions.
CCK radioimmunoassay
Samples are dissolved in assay diluent buffer as provided in the EURIA-CCK assay kit. The radioimmunoassay is performed using concentrations of standards ranging from 0 to 25 pmol/L. Standards and samples are incubated with antisera at 4°C for 48 hours prior to addition of 125I-CCK-8. Following a 96 hour incubation at 4°C, secondary antibody is added to all tubes and the radioactivity in the pellet is counted in a gamma counter.
CCK concentrations expressed as CCK-8 equivalents in pmol/L (fmol/mL) are calculated using non-linear regression analysis.
Assay characteristics
The sensitivity of the assay is sufficient to detect concentrations as low as 2 picomolar. Therefore, we use plasma samples of 2 mL in order to detect ~1 picomolar concentrations of blood CCK. In our hands the assay had the following characteristics:
- Intra-assay variation = 2.0 - 5.5%
- Inter-assay variation = 4.1 - 13.7%
References
EURIA-CCK
Cholecystokinin Radioimmunnoassay
Catalog Number: 013-RB-302
ALPCO Diagnostics
American Laboratory Products Company
P.O. Box 451
Windham, NH 03087
www.alpco.com
Choosing between the CCK bioassay and radioimmunoassay
The CCK bioassay was developed at a time when reliable CCK radioimmunoassays were lacking and only recently have CCK RIAs become commercially available. Now when measuring blood levels of CCK one is faced with a choice between the bioassay and a RIA. In our laboratory, we continue to use the bioassay for most measurements of plasma CCK. We find it reliable and rapid since results are available in one day. However, it does have several disadvantages. First, it is labor intensive and it is only available in laboratories where there are personnel experienced in working with pancreatic acinar preparations. Second, fewer samples can be measured relative to a RIA. In one day, we are able to assay ~20 samples with the bioassay. With replicates we are only able to assay 10 novel samples. Third, there is potential interference from drugs that could stimulate or (more likely) inhibit acinar secretion. With the RIA, many of the bioassay problems are obviated and it is possible to assay many more samples, although each sample must still be extracted from plasma. Extraction is also a labor-intensive process.
We prefer the RIA when measuring CCK secretion from isolated intestinal “I” cells or STC-1 cells in culture. The major advantage of the RIA for in vitro studies is the avoidance of interference from other hormones that may affect pancreatic secretion (e.g., secretin).
References
- Becker, H.D., Werner, M., and Schafmayer, A. Release of radioimmunologic cholecystokinin in human subjects. Am J Surg 147:124-129, 1984. PMID: 6691538
- Chang, T.M., and Chey, W.Y. Radioimmunoassay of cholecystokinin. Dig Dis Sci 28:456-468, 1983. PMID: 6839908
- Eberlein, G.A., Eysselein, V.E., Hesse, W.H., Goebell, H., Schaefer, M., and Reeve, J.R., Jr. Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol 253:G477-482, 1987. PMID: 3661709
- Eysselein, V.E., Eberlein, G.A., Hesse, W.H., Singer, M.V., Goebell, H., and Reeve, J.R., Jr. Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262:214-217, 1987. PMID: 3793725
- Hashimura, E., Shimizu, F., Nishino, T., Imagawa, K., Tateishi, K., and Hamaoka, T. Production of rabbit antibody specific for amino-terminal residues of cholecystokinin octapeptide (CCK-8) by selective suppression of cross-reactive antibody response. J Immunol Methods 55:375-387, 1982. PMID: 6300248
- Himeno, S., Tarui, S., Kanayama, S., Kuroshima, T., Shinomura, Y., Hayashi, C., Tateishi, K., Imagawa, K., Hashimura, E., and Hamaoka, T. Plasma cholecystokinin responses after ingestion of liquid meal and intraduodenal infusion of fat, amino acids, or hydrochloric acid in man: analysis with region specific radioimmunoassay. Am J Gastroenterol 78:703-707, 1983. PMID: 6637957
- Izzo, R.S., Brugge, W.R., and Praissman, M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept 9:21-34, 1984. PMID: 6095373
- Jansen, J.B., and Lamers, C.B. Radioimmunoassay of cholecystokinin in human tissue and plasma. Clin Chim Acta 131:305-316, 1983. PMID: 6883724
- Liddle, R.A. On the measurement of cholecystokinin. Clin Chem 44:903-904, 1998. PMID: 9590358
- Liddle, R.A., Goldfine, I.D., Rosen, M.S., Taplitz, R.A., and Williams, J.A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75:1144-1152, 1985. PMID: 2580857
- Liddle, R.A., Goldfine, I.D., and Williams, J.A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87:542-549, 1984. PMID: 6204904
- Liddle, R.A., Toskes, P.P., Horrow, J., Ghali, J., Dachman, A., and Stong, D. Lack of trophic pancreatic effects in humans with long-term administration of ximelagatran. Pancreas 32:205-210, 2006. PMID: 16552342
- Rehfeld, J.F. Accurate measurement of cholecystokinin in plasma. Clin Chem 44:991-1001, 1998. PMID: 9590372
- Tashiro, M., Samuelson, L.C., Liddle, R.A., and Williams, J.A. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286:G784-790, 2004. PMID: 14684381
- Turkelson, C.M., Dale, W.E., Reidelberger, R., and Solomon, T.E. Development of cholecystokinin radioimmunoassay using synthetic CCK-10 as immunogen. Regul Pept 15:205-217, 1986. PMID: 3786838
- Turkelson, C.M., Solomon, T.E., Bussjaeger, L., Turkelson, J., Ronk, M., Shively, J.E., Ho, F.J., and Reeve, J.R., Jr. Chemical characterization of rat cholecystokinin-58. Peptides 9:1255-1260, 1988. PMID: 3247248
- Williams, J.A. Isolation of rodent pancreatic acinar cells and acini by collagenase digestion. The Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2010.18, 2010.


