Methods Type:
Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2012.6
| Attachment | Size |
|---|---|
| 446.48 KB |
Different animal models of experimental acute pancreatitis induced by caerulein, choline deficient ethionine supplemented (CDE) diet, or sodium taurocholate have been developed. These models vary in severity and have been used by the investigators to either understand the pathophysiological mechanism of the disease or to study the effect of different therapeutic agents. Mizunuma et al (18) were first to report that intra-peritoneal (i.p.) administration of excessive doses of L-arginine (500 mg/100 g body weight) in rats selectively damage pancreatic acinar cells without any morphological change in islets of Langerhans or other organs. Based upon this observation, Tani et al (27) reported an L-arginine induced rat model of necrotizing acute pancreatitis. The dose used in this model was 500 mg/100 g. Doses higher than 500 mg/100 g body weight caused very high mortality. A single dose of 500 mg/100 g injected i.p. resulted in 70-80% of pancreatic acinar cell necrosis within 3 days. Since these observations, the model of L-arginine induced acute pancreatitis in rat has been used in different laboratories. The dose as well as frequency of administration has been varied. The different doses and frequencies of administration of L-arginine evaluated in rat by different investigators have been reviewed by Hegyi et al (15) and are summarized below:
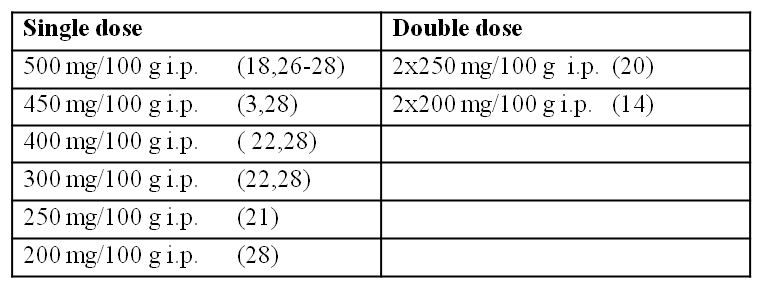
There are also reports in literature to indicate that even a single dose of 500 mg/100 g i.p. causes significant mortality in rats (20) while use of a double dose (2x250 mg/100 g at 1 h interval) reproducibly causes pancreatitis (20) without mortality. Some of the investigators have used overnight fasted animals while others used non-fasted animals for induction of L-arginine induced acute pancreatitis. In our studies, we have used non-fasted animals. We also reported an L-arginine induced pancreatitis model in mice using a two injection protocol (6). The L-arginine doses used in rats did not cause pancreatitis in mice. In mice a higher dose is required. This model has been used in a number of recent studies by different labs (1,8,10,13,16,19,24,25). In this Methods entry, we describe protocols for the induction of L-arginine induced acute pancreatitis in rats and mice.
1. Materials
1.1 L-arginine hydrochloride of highest purity available ( Sigma-Aldrich # A 5131)
1.2 Saline (0.15M NaCl solution) injectable grade, endotoxins free
1.3 Syringe filter (0.2 μm) 25mm
1.4 Syringe 5ml
1.5 Syringe 2ml
1.6 Needle 25 G 5/8” and 30 G ½”
1.7 pH meter
1.8 Rats (120-250 g)
1.9 Mice (25-28 g)
1.10 Balance for weighing animals
2. Method for Inducing L-Arginine Acute Pancreatitis in Rat
L-arginine hydrochloride solution: Prepare 20% L-arginine hydrochloride solution (Note 1) in saline and adjust the pH to 7.0. Filter through a syringe filter in a tissue culture hood into a sterile tube with a screw cap. We use 15 ml centrifuge tube (gamma sterilized, pyrogen, RNA, DNA free).
Induction of acute pancreatitis: Weigh the animal accurately and calculate the volume of L-arginine hydrochloride solution (20%) to be injected using a dose of 250 mg/100 g body weight (1.25 ml/100 g).
Fill the solution in a sterile 2 ml syringe with 25 G 5/8” needle and inject i.p. Put the animal in a clean cage with feed and water. We do the injection in a laminar air flow hood with filters. Wait for 1h and then administer the 2nd dose i.p. and return the animal to its cage. Rats become slow after the 2nd injection but gradually recover. Animals can be sacrificed from 6 h onward to evaluate changes in the pancreas, but peak histological changes are observed around 72 h after induction of pancreatitis.
Blood, pancreatic, and lung tissues are collected for evaluation of pancreatitis and associated lung injury.
Using this two injection protocol, we and others (20) have not observed mortality. In our earlier studies we and others (3,28) have used single injection protocol using dose 450 mg/ 100 g, which is associated with some mortality and is described below as an alternative protocol.
Single Injection Protocol: Weigh animals accurately and calculate the volume of 20% L-arginine hydrochloride solution required using a dose of 450 mg/100 g body weight. Inject i.p. the calculated volume of L-arginine hydrochloride solution and equivalent volume of normal saline in control animals. Put animal back in a clean cage with feed and water. Injected animals are kept under observation for 3-4 hours. Changes start appearing in the pancreas 6 h after the injection and reach to maximum in 72 h.
3. Method for Inducing L-Arginine Acute Pancreatitis in Mice
L-arginine hydrochloride solution: Prepare 8% L-arginine hydrochloride solution (Note 2) in saline and adjust the pH to 7.0. Filter through a syringe filter in a tissue culture hood into a sterile tube with screw cap. We use 15 ml centrifuge tube (gamma sterilized, pyrogen, RNA, DNA free).
Induction of acute pancreatitis: Weigh each animal accurately and calculate the volume of L-arginine solution to be injected using a L-arginine dose 400 mg/100 g body weight (1.5 ml/30 g). During the initial studies, we tried using higher concentrations of L-arginine hydrochloride solution to reduce the total volume of the injection but observed higher mortality. Administer the L-arginine solution intra-peritoneally using syringe with 30 G 1/2” needle followed by second i.p. injection after 1h. Control mice are injected with equivalent volume of normal saline. Mice become sluggish and their lethargy increases after the second injection. It takes 1-4 h to recover from this phase. The mortality associated with the model (5-7%), if any, occurs during this phase (Note 3). Plasma concentration of L-arginine increases after first i.p. injection and becomes further elevated after second injection, remaining high for up to 3 h (6).
Mice are kept under observation, allowed free access to feed and water, and sacrificed at 72 h. Blood is collected from heart using heparin treated syringes and is centrifuged at 4oC. Plasma is stored at -80oC for amylase, lipase, and cytokine analysis. Plasma amylase peaked at 72 h (Fig 1A). Other amino acids injected in a comparable amount had no effect on plasma amylase (Fig 1B).
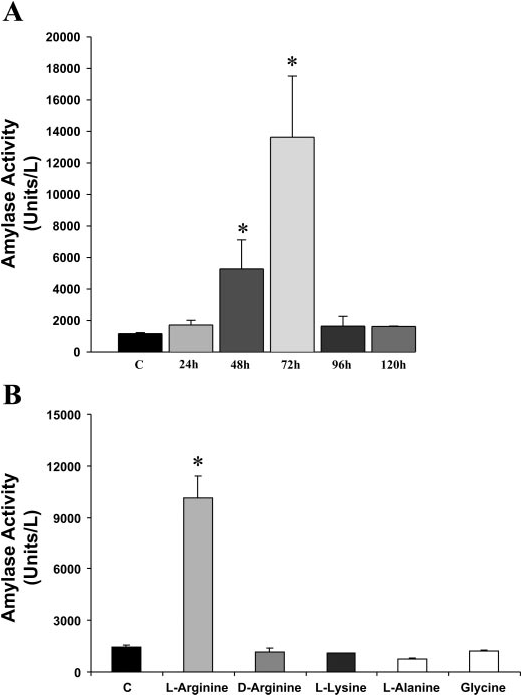
Fig.1 A: Plasma amylase activity in response to L-arginine administration (400 mg/100 g x 2) at different time points compared with control (C). B: Effect of administration of different amino acids on plasma amylase activity. Only L-arginine causes significant increase in plasma amylase activity in mice. *p < 0.05, n=6 for each time point (reproduced from Reference 6).
Pancreatic and lung tissues are collected for histology in 10% buffered formalin and snap frozen in liquid nitrogen for myeloperoxidase analysis. Pancreatic myeloperoxidase peaked at 72 h (Fig 2).
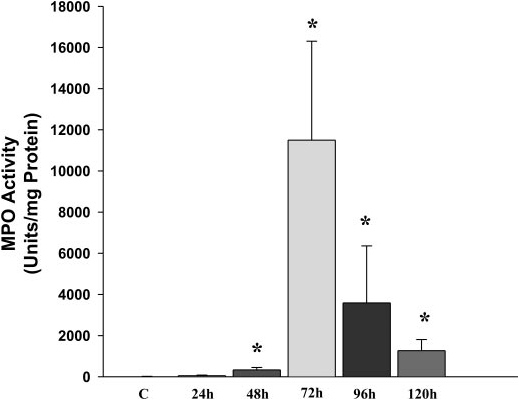
Fig. 2: Pancreatic myeloperoxidase (MPO) activity at different time points after administration of L-arginine compared with control (C). *p < 0.05 n >6 for each time point (reproduced from Reference 6).
Unlike rat models, in mice doses lower than 400 mg/100 g were not found effective for induction of acute pancreatitis (Fig 3).
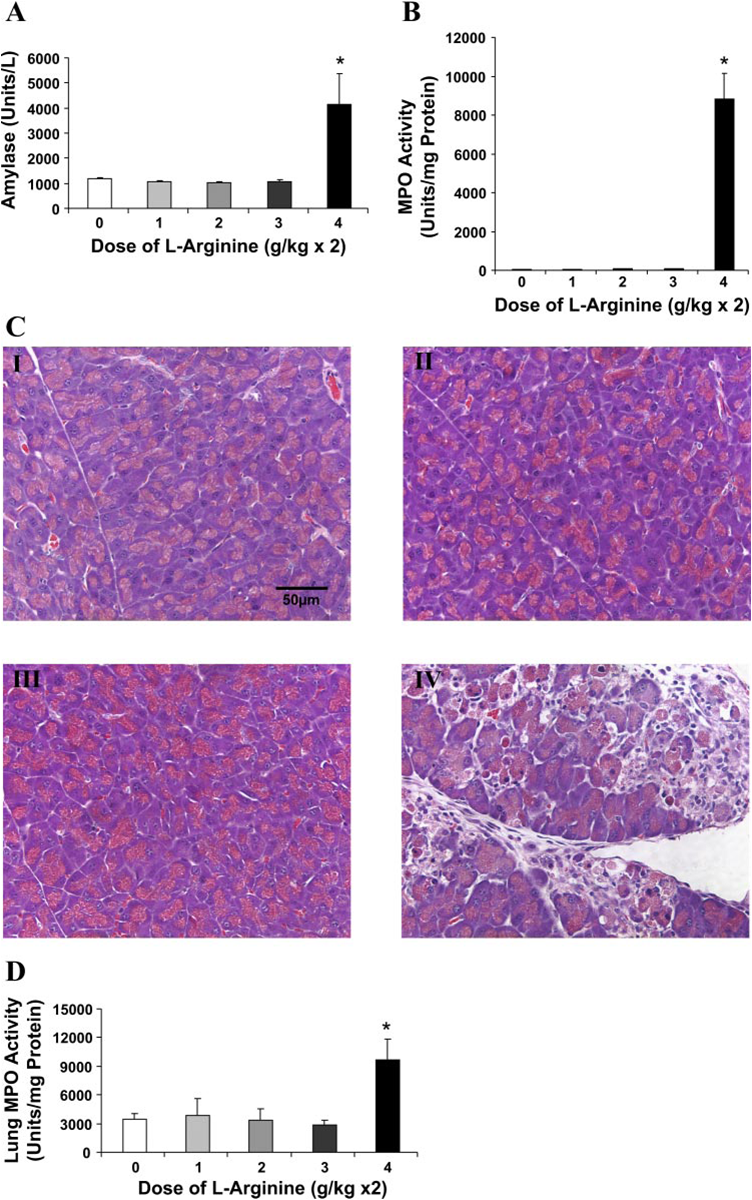
Fig. 3: Effect of different doses of L-arginine on plasma amylase, necrosis and lung myeloperoxidase in mice. Each dose was administered as 2 intraperitoneal injections 1 h apart. A: plasma amylase activity B: pancreatic myeloperoxidase C: representative H&E sections obtained from mouse pancreas in response to different doses of L-arginine administration after 72 h. Two doses of 100 mg/100 g (I), 200 mg/100 g (II), 300 mg/100 g (III) or 400 mg/100 g (IV). D: Lung myeloperoxidase activity. *p<0.05. n > 6 for each group (reproduced from Reference 6).
In our studies, we used C57BL/6 (inbred) and Balb/c (inbred) strains of mice and in subsequent studies from other labs C57BL/6 and FVB/N (inbred) strains have been used. We don’t have comparative experimental data using different strains (inbred and outbred) of mice for this model but expect some variation in susceptibility in view of different SPINK3 levels (28) and variability in arginine metabolism (9) in different strains.
We do not administer analgesics for pain during pancreatitis because of their potential for interference in development of inflammation. Preferred weight group is 25-28 g, because in this weight range animals recover better from initial complications associated with high dose L-arginine injections.
4. Mechanism of L-arginine induced pancreatitis
The mechanism of L-arginine induced pancreatitis is not clear. In the rat model of L-arginine- induced pancreatitis, partial inhibition of L-arginase activity, the enzyme which converts L-arginine to L-ornithine and urea, ameliorates pancreatitis indicating a role of one of its metabolites in induction of pancreatitis (2). Further at high dose, L-ornithine has also been reported to cause acute pancreatitis (23). L-arginine is also a substrate for nitric oxide synthase and is known to induce nitrostative and oxidative stress. There is some evidence to support that these pathways might also contribute to pancreatic injury in this model of pancreatitis. L-arginine pancreatitis is ameliorated by treatments directed at reducing oxidative stress (11,12) Lechin and Vander Dijs (17) suggested that L-arginine might induce pancreatitis through activation of autonomous nervous system. This was based on the observation in their studies that a small oral dose of L-arginine (50mg) is able to provide significant change in plasma neurotransmitters. These could cause hypersecretion, which can’t be drained through pancreatic duct. This is a less plausible mechanism in view of the facts that (a) the dose used in this model is very high, (b) low doses do not cause pancreatitis, and (c) there is inhibition in secretion rather than hypersecretion in response to caerulein stimulation from acinar cells prepared from animals, in which pancreatitis was induced using L-arginine (7).
Bohus et al (5) evaluated metabolic changes at different time points after administration of low (100 mg/100 g) and high dose (400 mg/100 g) of L-arginine to rat. At higher dose (relevant to arginine model), initial phase (0-8 h) involved changes in the urea cycle and transamination, indicating homeostatic response to detoxify excess ammonia generated from arginine catabolism. Also i.p. administration of chloride salts of L-arginine resulted in metabolic acidosis and the mean pH of urine samples was lower than control for up to 168 h reaching the most acidic values (pH=5.5-6.0) at 24 and 48 h. Serum urea nitrogen levels were significantly elevated at 24 h indicating deamination being directed into increased urea synthesis. Urea nitrogen returned to control levels by 48 h due to clearance of L-arginine challenge. High dose animals faced a greater excess of arginine than the low dose group. There was enhanced activity of the urea cycle and transamination as well as higher excretion of L-arginine. Metabolic acidosis itself can enhance secretagogue–induced zymogen activation (4).
Although the exact mechanism of L-arginine pancreatitis is not clear, the available evidence indicates that its metabolites, oxidative stress and metabolic acidosis contribute to the pancreatic injury in this model.
5. Notes
- Weigh 2 g L-arginine hydrochloride dissolve in 8ml saline, adjust pH to 7.0 and make volume to 10 ml with saline. We prepare fresh solution on the day of injections.
- Weigh 800 mg L-arginine hydrochloride and dissolve in 8 ml saline, adjust pH to 7.0 and make volume to 10 ml with saline. Prepare fresh before injection.
- The effective dose is very close to the toxic dose.
6. References
- Abdulla A, Awla D, Hartman H, Rahman H, Jeppsson B, Regner S, Thorlacius H. Role of platelets in experimental acute pancreatitis. Br J Surg., 98: 93-103, 2011. PMID: 20882560
- Biczo G, Hegyi P, Berczi S, Dosa S, Hracsko Z, Varga I, Ivanyi B, Venglovecz V, Wittmann T, Takacs T, Rakonczay Z . Inhibition of arginase activity ameliorates L-arginine induced pancreatitis in rats. Pancreas, 39: 868-874, 2010. PMID: 20697209
- Bhagat L, Singh VP, Dawra RK, Saluja AK. Sodium arsenite induces heat shock protein 70 expression and protects against secretagogue-induced trypsinogen and NF-kappaB activation. J Cell Physiol., 215: 37-46, 2008. PMID: 17941083
- Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, Thrower EC, Gorelick FS. Reducing extracellular pH sensitizes the acinar cell to secretagogogue-induced pancreatitis responses in rats. Gastroenterology, 137:1083-1092, 2009. PMID: 19454288
- Bohus E, Coen M, Keun HC, Ebbels TM, Beckonert O, Lindon JC, Holmes E, Noszal B, Nicholson JK . Temporal metabolomics modeling of L-arginine induced exocrine pancreatitis. J Proteome Res., 7: 4435-4445, 2008. PMID: 18710274
- Dawra R, Sharif R, Phillips P, Dudeja V, Dhaulakhandi D, Saluja A. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol., 292: G1009-G1018, 2007. PMID: 17170029
- Dawra R, Saluja A. Reply to lechin and van der Dijs. Am J Physiol Gastrointest Liver Physiol., 294: G1452, 2008. PMID: 18541710
- Dahlhoff M, Alqül H, Siveke JT, Lesina M, Wanke R, Wartmann T, Halangk W, Schmid RM, Wolf E, Schneider MR. Betacellulin protects from pancreatitis by activating stress-activated protein kinase. Gastroenterology, 138: 1585-1594, 2010. PMID: 20038432
- Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouède S, Boucher JL, Wilson KT, Veyret B, Gobert AP. Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J Immunol., 172: 6298-6303, 2004. PMID: 15128819
- Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, Stevenson DK, Butcher EC, Omary MB. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut, 60: 671-679, 2011. PMID: 21159893
- Hardman J, Jamdar S, Shields C, McMahon R, Redmond HP, Siriwardena AK. Intravenous selenium modulates L-arginine induced experimental acute pancreatitis. JOP., 6:431-437, 2005. PMID: 16186664
- Hardman J, Shields C, Schofield D, MacMahon R, Redmond HP, Siriwardena AK. Intravenous antioxidant modulation of end-organ damage in L-arginine-induced experimental acute pancreatitis. Pancreatology, 5: 380-386, 2005. PMID: 15980666
- Hartman H, Abdulla A, Awla D, Lindkvist B, Jeppsson B, Thorlacius H, Regner S. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg., 99:246-255, 2012. PMID: 22109627
- Hegyi P, Takács T, Varga IS, Tiszlavicz L, Hai DQ, Hegi P, Matkovics B, Lonovics J. Spontaneous and cholecystokinin-octapeptide-promoted regeneration of the pancreas following L-arginine-induced pancreatitis in rat. Int J Pancreatol., 22:193-200, 1997. PMID: 9444550
- Hegyi P, Rakonczay Z, Sári R, Góg C, Lonovics J, Takács T, Czakó L. L-arginine-induced experimental pancreatitis. World J. Gastroenterol.; 10: 2003-2009, 2004. PMID: 15237423
- Hu G, Shen J, Cheng L, Guo C, Xu X, Wang F, Huang L, Yang L, He M, Xiang D, Zhu S, Wu M, Yu Y, Han W, Wang X. Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut, 60: 820-828, 2011. PMID: 21193457
- Lechin F, van der Dijs B. Arginine–induced pancreatitis: involvement of the autonomic nervous system? Am J Physiol Gastrointest Liver Physiol., 294: G1450-G1451, 2008. PMID: 18541710
- Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J Nutr., 114: 467-471, 1984. PMID: 6199486
- Molero X, Vaquero EC, Flández M, González AM, Oritz MA, Cibrián-Uhalte E, Servita JM, Merlos A, Juanpere N, Massumi M, Skoudy A, Macdonald R, Ferrer J, Real FX. Gene expression dynamics after murine pancreatitis unveils novel roles for Hnf1{alpha} in acinar cell homeostasis. Gut, Sep 23, 2011. PMID: 21948943
- Moreira M, Matias JE, Souza CJ, Nicoluzzi JE, Caron PE, Repka JC. Action of tacrolimus in arginine induced experimental acute pancreatitis. Rev Col Bras Cir., 38: 260-265, 2011. PMID: 21971860
- Pozsár J, Schwab R, Simon K, Fekete L, Orgován G, Pap A. Effect of endotoxin administration on the severity of acute pancreatitis in two experimental models. Int J Pancreatol., 22: 31-37, 1997. PMID: 9387022
- Rakonczay Z Jr, Iványi B, Varga I, Boros I, Jednákovits A, Németh I, Lonovics J, Takács T. Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. Free Radic Biol Med., 32: 1283-1292, 2002. PMID: 12057766
- Rakonczay Z Jr, Hegyi P, Dósa S, Iványi B, Jármay K, Biczó G, Hracskó Z, Varga IS, Karg E, Kaszaki J, Varró A, Lonovics J, Boros I, Gukovsky I, Gukovskaya AS, Pandol SJ, Takács T. A new severe acute necrotizing pancreatitis model induced by L-ornithine in rats. Crit Care Med., 36: 2117-2123, 2008. PMID: 18594222
- Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata, N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, Takeda K, Hozawa S, Yoshimura A, Hibi T. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulean-induced pancreatitis in mice. Gastroenterology, Jan 13, 2012. PMID: 22248664
- Shen JQ, Shen J, Wang XP. Expression of insulin-like growth factor binding protein-4 (IGFBP-4) in acute pancreatitis induced by L-arginine in mice. Acta Histochem., Aug 10, 2011. PMID: 21839495
- Shields CJ, Winter DC, Sookhai S, Ryan L, Kirwan WO, Redmond HP. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br J Surg., 87: 1336-1340, 2000. PMID: 11044157
- Tani S, Itoh H, Okabayashi Y, Nakamura T, Fuji M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci., 35: 367-374, 1990. PMID: 2307082
- Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion, 65: 118-126, 2002. PMID: 12021485
- Wang J, Ohmuraya M, Suvama K, Hirota M, Ozaki N, Baba H, Nakagata N, Araki K, Yamamura K. Relationship of strain-dependent susceptibility to experimentally induced acute pancreatitis with regulation of Prss1 and Spink3 expression. Lab Invest., 90: 654-664, 2010. PMID: 20157294


