Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2021.05
| Attachment | Size |
|---|---|
| 1.2 MB |
I. Introduction
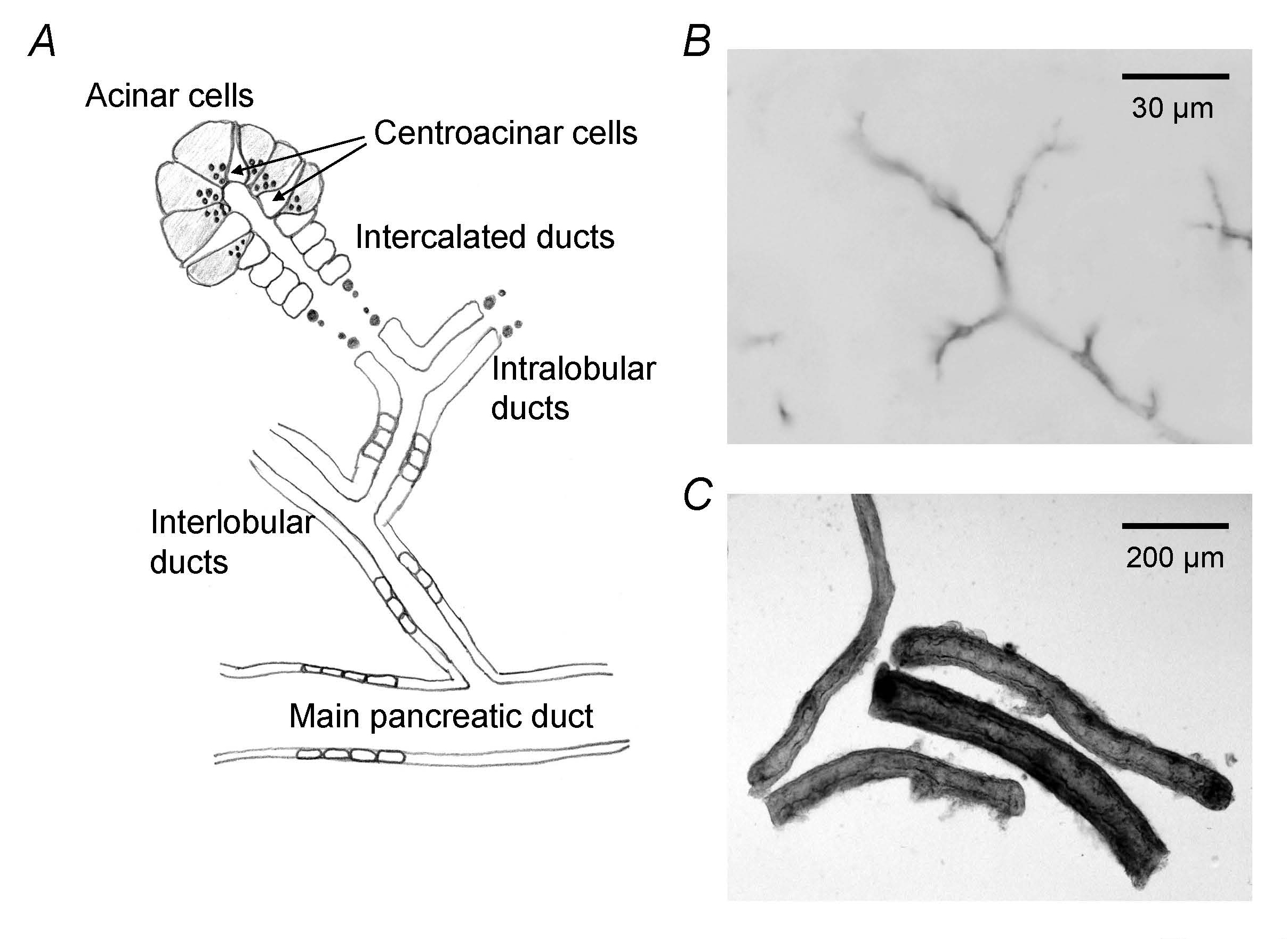
Pancreatic juice is the product of 2 distinct secretory processes. Enzymes are secreted by exocytosis from acinar cells. Fluid and electrolyte secretion is achieved primarily by the vectorial transport of ions across the ductal epithelium accompanied by water in isotonic proportions (Figure 1A). Each day, the human pancreas delivers 6–20 g of digestive enzymes to the duodenum in approximately 2.5 liters of HCO3−-rich fluid. The HCO3−-rich fluid acts as a vehicle for transporting enzymes to the duodenum where the HCO3− neutralizes gastric acid. Moreover, pancreatic HCO3− secretion is thought to aid disaggregation of secreted enzymes following their exocytosis in the lumen of pancreatic duct.
Figure 1. Pancreatic duct system. (A) Schematic architecture of pancreatic ductal tree. Centroacinar cells belong to duct cells and represent the terminal cells of the ductal tree. (B) Immunostaining of CFTR in guinea-pig pancreas indicates apical membrane of pancreatic ductal epithelium. (C) Interlobular duct segments isolated from guinea-pig pancreas. (Adapted from reference (19)).
II. Species-Dependent Regulation of Pancreatic Fluid and Electrolyte Secretion
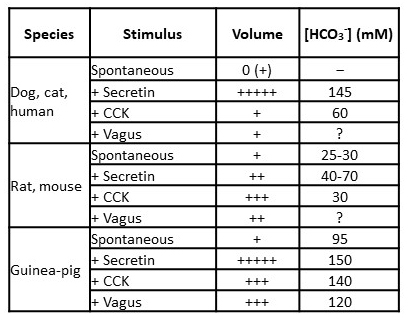
The regulation of pancreatic fluid and electrolyte secretion and the volume and composition of the secreted fluid differ considerably from species to species (Table 1) (19). In all species, secretin evokes the secretion of HCO3−-rich pancreatic juice. However, the amount of fluid is small in the rat and mouse (approximately fivefold less than in the cat, per gram of tissue). Maximal HCO3− concentration ([HCO3−]) reaches 130 mM or more in all species except the rat and mouse, in which 70 and 40 mM are the values. The secreted fluid is nearly isotonic ([HCO3-] + [Cl-] = 150-160 mM) because of the high transepithelial water permeability and the Na+ conductance of the paracellular pathway.
Table 1. Species-dependent patterns of pancreatic HCO3- and fluid secretion. This table shows the response to stimuli given alone. Potentiation often occurs when stimuli are given together. Most data were obtained from studies on anesthetized animals. Quantitative differences may occur in conscious animals, especially in the rat, in which secretion is increased fivefold in conscious animals. (Adapted from reference (19)).
[HCO3-] in human pancreatic juice reaches ~140 mM under stimulation. It is generally thought that most of the HCO3- secretion originates from epithelial cells lining the proximal pancreatic ducts (centroacinar cells, intralobular ducts, and small interlobular ducts) (Figure 1A). Pancreatic HCO3- secretion depends on the activity of cystic fibrosis transmembrane conductance regulator (CFTR) anion channel localized in the apical membrane of pancreatic duct cells (Figure 1B). While acinar cells add a small volume of Cl- and protein-rich secretion to the lumen, luminal [HCO3-] quickly increases with time and distance along the duct as a result of HCO3- secretion by ductal epithelium. Thus, pancreatic duct cells can transport HCO3- against 5-6-fold concentration gradients.
III. Vectorial Transport in Isolated Pancreatic Duct
Studies using isolated interlobular pancreatic ducts from guinea-pig pancreas (Figure 1C) demonstrated that pancreatic duct cell is capable to secrete HCO3− into already HCO3−-rich fluid under cAMP stimulation (Figure 2A) (15, 23). The isolated pancreatic ducts respond to physiological concentrations (1-10 pM) of secretin (Figure 2B) (15, 45).
Figure 2. Fluid and HCO3- secretion by interlobular duct segments isolated from guinea-pig pancreas. (A) When isolated segments of interlobular pancreatic ducts are cultured overnight, both ends of the ducts seal spontaneously. The lumen of sealed ducts is micropunctured and membrane-impermeable dextran conjugate of the pH sensor BCECF is injected. Time course changes in luminal pH (upper panel) and fluid secretory rates, determined from expansion speed of the lumen (lower panel), in ducts filled with high-HCO3- solution (125 mM HCO3−-24 mM Cl-, pH ~8.2), before and after cAMP stimulation (0.1 μM forskolin), are shown (means ± SE of 4 experiments). (Adapted from reference (23)). Before cAMP stimulation, there is a slight decrease of luminal pH accompanied by fluid secretion (likely Cl--rich). Upon stimulation, luminal pH starts to rise, which is accompanied by increase of fluid secretion (HCO3−-rich). (B) Effects of secretin concentration on the rate of fluid secretion into the lumen of sealed ducts (means ± SE of 5 experiments). (Adapted from reference (45)).
Measurement of intracellular pH in luminally-microperfused pancreatic duct demonstrate a polarity which is necessary to achieve vectorial transport of HCO3− (14). While basolaterally-applied HCO3− easily gains access to the cell, intraluminally-applied HCO3− does not. HCO3− accumulation across the basolateral membrane is largely mediated by a electrogenic Na+-coupled HCO3− transporter (NBCe1-B, SLC4A4) and the contribution of Na+-H+ exchanger 1 (NHE1, SLC9A1) is small (Figure 3) (19). Although the CFTR-dependent HCO3− permeability of the apical membrane is large enough to mediate the observed HCO3− secretion (16), the cell is relatively hyperpolarized (17) and the electrochemical gradient for HCO3− is outwardly-directed (does not allow entry of luminal HCO3−) (Figure 3).
Figure 3. A model of HCO3− secretion by guinea-pig pancreatic duct cell. (A) Unstimulated duct cell whose apical membrane faces low-HCO3−, high-Cl- pancreatic juice. Accumulation of HCO3− in the cell is achieved mainly by basolateral NBCe1, while the contribution of NHE1 is small. Some HCO3− is lost via basolateral AE2. HCO3− secretion across the apical membrane is mediated by SLC26A3/6 and CFTR. CFTR mediates both Cl- and HCO3− secretion. (B) Cyclic AMP-stimulated duct cell of which the apical membrane faces high-HCO3−, low-Cl- pancreatic juice. NBCe1, SLC26A3/6, and CFTR are activated, while AE2 is inhibited. At the basolateral membrane, HCO3− accumulation via NBCe1 is increased, while Cl- uptake (and HCO3− loss) via AE2 is reduced. As luminal [HCO3−] increases, the contribution of SLC26A3/6 becomes smaller and CFTR mediates most of HCO3− secretion. The Na+:HCO3− stoichiometry of NBCe1 is 1:2. SLC26A3 and SLC26A6 are reported to be electrogenic with opposite polarities: SLC26A3 mediates 2Cl--1HCO3− exchange, while SLC26A6 mediates 1Cl--2HCO3− exchange. (Adapted from reference (19)).
Cholinergic stimulation also stimulates HCO3− secretion in guinea-pig pancreatic duct (18). Ca2+-activated Cl- channels (CaCCs) in the apical membrane (1) instead of CFTR are thought to be involved in HCO3− secretion. The molecular identity of CaCCs in pancreatic duct cell is not known at present (20).
IV. Regulation of Pancreatic Ductal Secretion
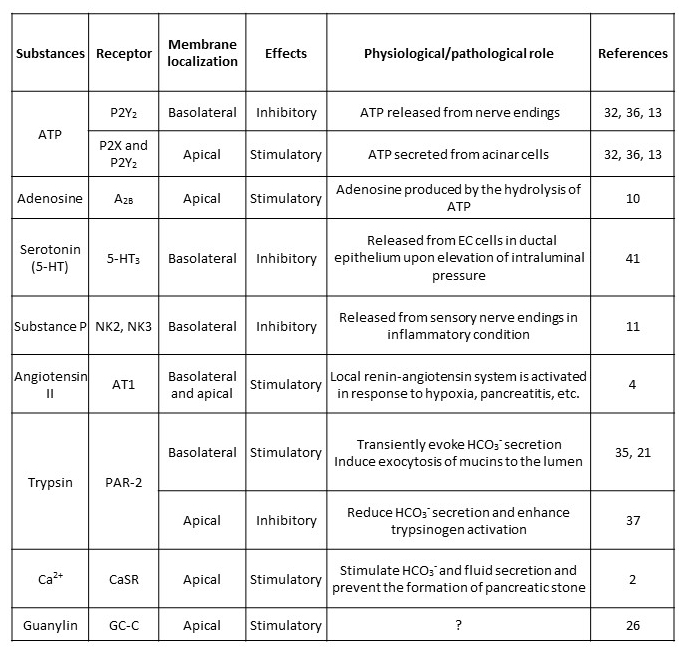
This section focuses on the regulatory mechanisms of pancreatic juice secretion which directly act on pancreatic duct cells. In vitro studies using isolated pancreatic ducts or cell lines derived from human pancreatic ductal adenocarcinoma (Capan-1, PANC-1, CFPAC-1 from a patient with cystic fibrosis homozygous for F508del) demonstrated the presence of receptors of various hormones and neurotransmitters and their effects on intracellular signaling, ion transport, and HCO3−/fluid secretion (1). Enhancement of HCO3−/fluid secretion is usually associated with accumulation of intracellular cAMP or elevation of intracellular Ca2+ ([Ca2+]i). However, we should note that elevation of [Ca2+]i is not necessarily associated with the increase in HCO3−/fluid secretion and that pancreatic ductal regulation is also regulated by various intraluminal substances (Table 2).
Table 2. Local regulation of pancreatic ductal secretion. This table shows the list of substances which are thought to be locally released in the pancreas and directly act on pancreatic duct cells.
The major regulators are secretin, vasoactive intestinal peptide (VIP), and acetylcholine which increase intracellular Ca2+/cAMP and stimulate HCO3− and fluid secretion. Cholecystokinin (CCK) and bombesin stimulate not only enzyme secretion by acinar cells but also HCO3− and fluid secretion by duct cells (42). In isolated vascularly-perfused guinea-pig pancreas, arginine vasopressin (AVP) increases the vascular resistance and inhibited fluid secretion (22). But AVP also directly acts on pancreatic duct cell and inhibits fluid secretion which is associated with [Ca2+]i elevation. Reduction of pancreatic juice secretion by AVP may be physiologically important for body fluid conservation.
The long-term regulation of HCO3- and fluid secretion by pancreatic duct cells is thought to be under neurohormonal control and mediated by receptors localized in the basolateral membranes. However, in some occasions, the ion composition and pH of the luminal fluid as well as intraductal pressure needs to be controlled to avoid mechanical injury to ductal epithelium, autoactivation of trypsin, or precipitation of pancreatic stone protein and CaCO3 in the lumen. Table 2 summarizes the candidate list of local (stimulatory and inhibitory) regulators which are locally produced in the pancreas and play autocrine or paracrine roles.
Pancreatic duct epithelium expresses purinergic (P2Y and P2X) and adenosine receptors (10, 32, 36). Thus, pancreatic ductal secretion is likely influenced by luminal ATP and ATPase (ectonucleotidase) secreted by acinar cells together with the exocytotic release of digestive enzymes (24, 40) as well as basolateral ATP released as neurotransmitter. In isolated pancreatic ducts, ATP causes [Ca2+]i increase when applied to either the apical or basolateral membrane (13). However, apical and basolateral purinoceptors have opposite effects on secretion: luminally-applied ATP enhances HCO3− and fluid secretion while basolaterally-applied ATP inhibits secretion. Luminal adenosine activates CFTR in Capan-1 cells (10).
Figure 4. Serotonin (5-HT) inhibits pancreatic fluid secretion in an autocrine manner. (A) Effects of serotonin (5-HT 0.1 μM) on secretin (1 nM)-stimulated fluid secretion by isolated guinea-pig pancreatic ducts (means ± SE of 4 experiments). (B) Effects of small elevations of intraductal pressure and 5-HT3 receptor antagonist (granisetron 40 μg/kg/h) on in vivo secretin (1 μg/kg/h)-stimulated fluid secretion from guinea-pig pancreas (means ± SE of 4 experiments). (C) A hypothetical mechanism. EC cells in the duct epithelium sense the elevation of intraductal pressure and release 5-HT into the interstitium. The released 5-HT binds to 5-HT3 receptor in the basolateral membrane of neighboring duct cells, which facilitates Na+ entry into the cell. That reduces the inward gradient of Na+, which is necessary for intracellular HCO3- accumulation. (D) A 5-HT-immunoreactive cell embedded in pancreatic duct epithelium. (Adapted from reference (41)).
5-hydroxytryptamine (5-HT, serotonin)-immunoreactive cells with the morphological characteristics of enterochromaffin cells (EC cells) are found scattered in the pancreatic duct epithelium (Figure 4D) (41) These cells may sense the elevation of intraductal pressure (as EC cells do in the intestine), and the released 5-HT may inhibit fluid secretion by pancreatic duct (Figure 4C). In fact, basolateral application of 5-HT at relatively low concentrations (IC50: ~30 nM), inhibited fluid secretion (~75%) by isolated pancreatic ducts (Figure 4A). A small elevation (+ 3 cm H2O) of intraluminal pressure reversibly reduced pancreatic fluid secretion in vivo (anesthetized guinea-pig), and the effect was attenuated by intravenous granisetron, a 5-HT3 receptor antagonist (Figure 4B). The intraductal pressure of the human pancreas increases after feeding by as much as ~20 cm of H2O (8). The pressure is elevated in some patients with chronic pancreatitis. Thus 5-HT may regulate pancreatic fluid secretion under physiological and pathological conditions.
During the course of acute and chronic pancreatitis, substance P and calcitonin gene-related peptide (CGRP) are released from intrapancreatic endings of sensory nerves, which cause vasodilatation and mediate hyperalgesia (12). Substance P strongly inhibits HCO3− and fluid secretion by isolated pancreatic duct (11). Pancreatic fluid hypersecretion was observed during the early stage of caerulein-induced experimental acute pancreatitis in rats (6). While it is not known whether such fluid hypersecretion occurs in human acute pancreatitis, substance P may protect the pancreas by inhibiting ductal fluid secretion
A local intrapancreatic renin-angiotensin system (RAS) is activated in response to hypoxia, pancreatitis, etc. and involved in apoptosis and fibrosis (30). Inhibition of RAS in stellate cells attenuates pancreatic fibrosis in a rat model of chronic pancreatitis (27). Angiotensin II (either luminal or basolateral) induces anion secretion in CFPAC-1 cells which is associated with [Ca2+]i elevation (4). Thus, the activation of intrapancreatic RAS may increase the volume of pancreatic juice.
The membrane localization of proteinase-activated receptor-2 (PAR-2) in pancreatic duct cells and the effect of trypsin on HCO3− secretion are controversial. Basolateral trypsin causes [Ca2+]i increase and induces HCO3− secretion via activation of CFTR-dependent apical Cl--HCO3- exchange in Capan-1 cells (35) and stimulates exocytosis of mucins to the lumen in canine pancreatic duct cells (21). On the contrary, apical trypsin causes [Ca2+]i increase but reduces HCO3− secretion by inhibition of CFTR and apical Cl--HCO3- exchange in guinea-pig pancreatic duct cells (37). Consequent acidification of luminal pH may promote the autoactivation of trypsin. Those controversial data may be related to the conflicting effects of genetic PAR-2 deletion on experimental pancreatitis (29). While caerulein-induced pancreatitis is worse in PAR-2-/- mice, taurocholate-induced pancreatitis is milder in PAR-2-/- mice.
Ca2+-sensing receptor (CaSR) is localized to the apical membrane of human and rat pancreatic duct cells (2). Luminal application of Gd3+, a CaSR agonist induces [Ca2+]i elevation and stimulates HCO3- secretion in rat pancreatic duct. [HCO3-] in human pancreatic juice reaches ~140 mM and [CO32-] is estimated to be 0.03~1 mM. The pancreatic juice also contains millimolar quantities of Ca2+, which are released from acinar cells along with digestive enzymes. CaSR-mediated fluid secretion by pancreatic duct may be crucial to prevent precipitation of CaCO3 by dilution. Some studies suggest that CaSR variants increase the risk of chronic pancreatitis (28).
In human pancreas, guanylin is specifically localized to centroacinar cells and proximal duct cells (26). Guanylin activates CFTR via cGMP in Capan-1 cells. Thus, guanylin may regulate ductal cell secretion in a luminocrine mode, although the signal to induce guanylin secretion is not known.
V. Pancreatic Ductal Secretion and Lifestyle Disease
A. Effects of ethanol
Ethanol abuse is the leading cause of acute and chronic pancreatitis. The more the daily amount of alcohol drinking, the larger the risk of developing chronic pancreatitis (31). However, there is no known threshold dose of ethanol that induces chronic pancreatitis and patients drinking even small quantities of ethanol (1-20 g per day) run a higher risk. Studies using isolated pancreatic ducts from guinea-pig show that low and high concentrations of ethanol have opposite effects on fluid secretion. While low concentration of ethanol (0.1-30 mM relevant to normal drinking conditions) enhance fluid secretion (9, 45), high concentrations of ethanol (100 mM relevant to lethal level) reduce fluid secretion (33).
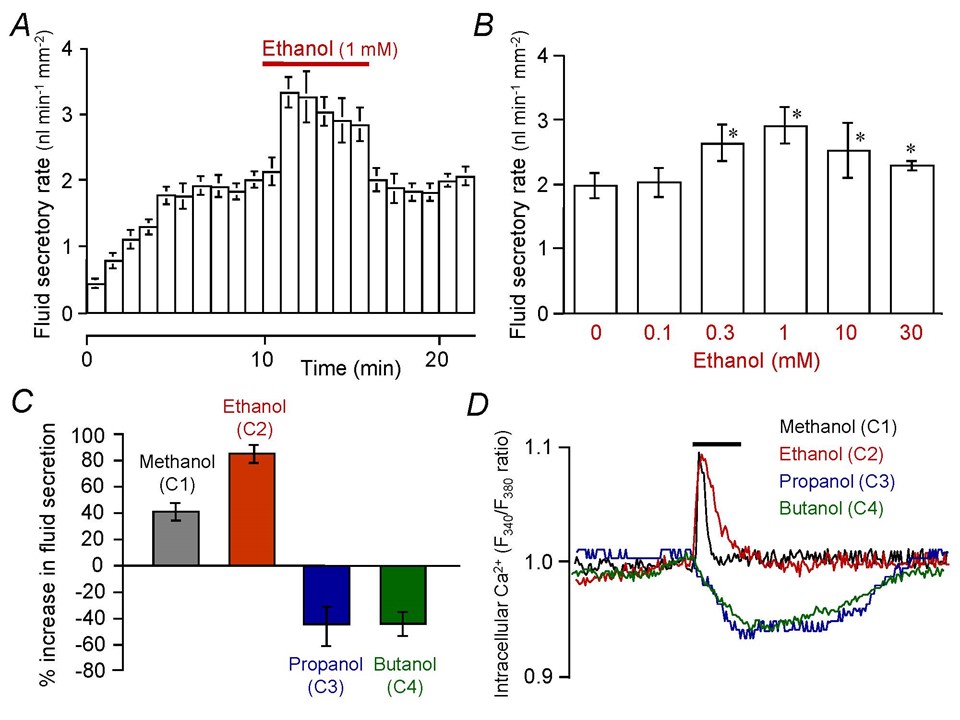
The objective effects of ethanol on the central nervous system (CNS) appear at a blood ethanol level of ~5 mM, and they are attributed to the modulation of ion channels (25). Ethanol reversibly and strongly augments secretin-stimulated fluid secretion in guinea-pig pancreatic duct (Figure 5A) where the maximal augmentative effect is achieved with 1 mM ethanol (Figure 5B) (45). The augmentation of fluid secretion involves a transient increase of [Ca2+]i (Figure 5D) likely via the activation of plasma membrane Ca2+ channels. The effects of n-alcohols on pancreatic duct cells are dependent on the length of alkyl chain. While methanol (C1) and ethanol (C2) augment fluid secretion and induce a transient [Ca2+]i increase, propanol (C3) and butanol (C4) inhibit fluid secretion and reduce [Ca2+]i (Figure 5C and D) (9). The observation is similar to the so-called “cutoff” effects of n-alcohols on neurotransmitter-gated ion channels in CNS neurons that increase in potency, with an increasing alkyl chain length of n-alcohol up to a point but then disappears with a further increase in chain length (25). Concerning the pathogenesis of alcoholic chronic pancreatitis, the ductal fluid hypersecretion by ethanol may raise the intraductal pressure when the flow of pancreatic juice is blocked by the presence of highly viscous juice or protein plugs.
Figure 5. Effects of low concentrations of alcohol on fluid secretion and intracellular Ca2+ response in isolated pancreatic ducts. (A) Rapid and reversible effects of ethanol on secretin (0.3 nM)-stimulated fluid secretion in isolated guinea-pig pancreatic ducts (means ± SE of 6 experiments). (B) Effects of various concentrations of ethanol (0.1-30 mM) on secretin (1 nM)-stimulated fluid secretion (means ± SE of 4-6 experiments). (C) Effects of n-alcohols (C1 methanol, C2 ethanol, C3 propanol, C4 butanol at 1 mM) on secretin-stimulated fluid secretion (means ± SE of 4 experiments). (D) Effects of n-alcohols (C1-C4) on intracellular Ca2+ concentration under secretin stimulation. Each trace is representative of 4 experiments. (Adapted from references (9, 45)).
Ethanol (100 mM) inhibits cAMP-stimulated CFTR currents, apical Cl--HCO3- exchange, and fluid secretion in guinea-pig pancreatic ductal epithelial cells (33). Moreover, prolonged treatment with 100 mM ethanol decreases membrane expression of CFTR. Those effects of ethanol involve sustained elevation of [Ca2+]i and depletion of ATP, which is probably associated with the pathogenesis of alcoholic pancreatitis.
B. Effects of glucose
Pancreatic exocrine dysfunction is frequently found in patients with type 1 and type 2 diabetes mellitus. Both enzyme secretion from acinar cells and fluid/HCO3- secretion by duct cells are impaired. It was reported that exposure to high glucose (for 72 h) activates polyol metabolism and decreases the activity of Na+,K+-ATPase in Capan-1 cells (3). The resultant elevation of [Na+]i would attenuate the inward Na+ gradient across the basolateral membrane, which reduces HCO3- uptake via Na+-HCO3- cotransport and Na+-H+ exchange (Figure 3). This leads to a reduction of fluid secretion.
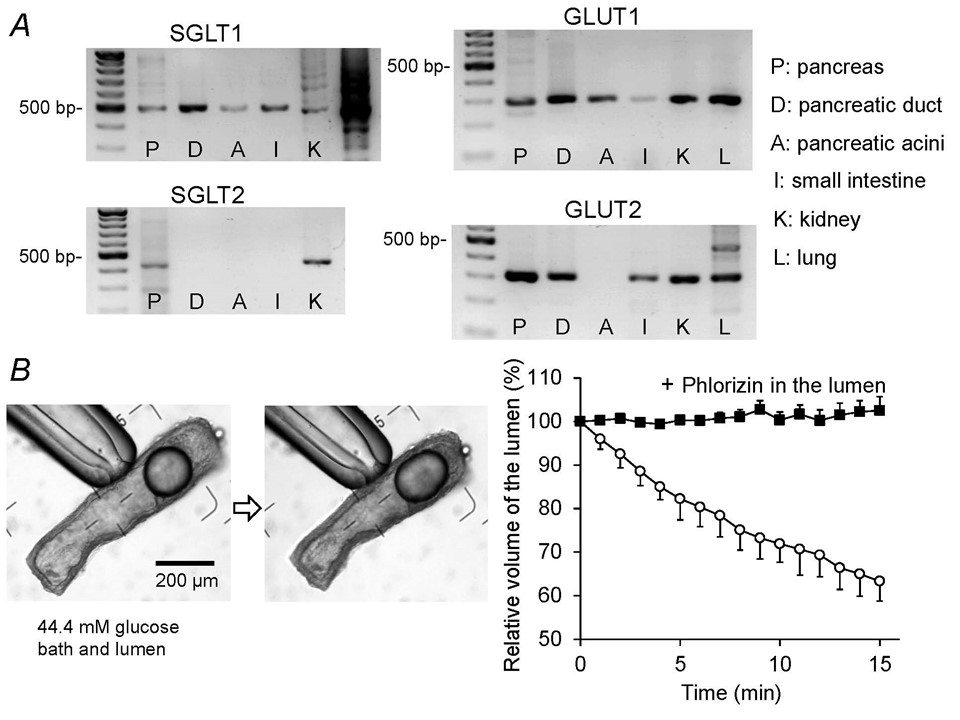
Even acute exposure to high glucose concentrations (15 min) inhibits fluid secretion and reduces basolateral HCO3- uptake in secretin-stimulated rat pancreatic ducts (7). This inhibition is most likely attributed to transepithelial absorption of glucose. Rat interlobular pancreatic ducts express Na+-glucose cotransporter (SGLT1) and glucose transporters (GLUT1 and GLUT2) (Figure 6A), and absorb luminal glucose iso-osmotically (Figure 6B). The absorption of luminal glucose is abolished by phlorizin, an inhibitor of SGLT1 in the lumen. Absorption of luminal Na+ and glucose via SGLT1 would increase [Na+]i and depolarize the apical membrane. [Na+]i elevation reduces HCO3- uptake and apical depolarization reduces the driving force for HCO3- secretion via CFTR, both of which leads to a reduction of fluid secretion.
Figure 6. Expression of glucose transporters and transepithelial glucose absorption by isolated pancreatic ducts. (A) mRNA expression of glucose transporters (SGLT1, SGLT2, GLUT1, GLUT2) in the pancreas (P), pancreatic duct (D), pancreatic acini (A), small intestine (I), kidney (K), and lung (L) of the rat. (B) Time course shrinkage of the lumen indicates transepithelial absorption of luminal glucose by isolated rat pancreatic ducts. The lumen is filled with the HCO3--free HEPES-buffered solution containing high glucose (44.4 mM). The bath is also perfused with the HCO3--free HEPES-buffered solution containing 44.4 mM glucose. The left panel shows images of a duct at the beginning and end of a representative experiment (15 min) showing 33% shrinkage of the lumen. Time-course changes of the luminal volume (right panel) demonstrate that phlorizin (0.5 mM) in the lumen completely inhibits glucose absorption (means ± SE of 4 experiments). (Adapted from reference (7)).
The glucose concentration in human pancreatic juice (0.5~1 mM) is much lower than in plasma. Under physiological conditions, pancreatic duct epithelium probably absorbs luminal glucose via apical SGLT1 to maintain the glucose concentration at a low level in the pancreatic juice.
VI. Computer Simulation of Pancreatic Ductal Secretion
On the basis of previous modelling studies (39, 43), a new computational model has been developed as a set of simultaneous ordinary differential equations in MATLAB (MathWorks, Natick, MA, USA) using the Simulink interface to provide a modular structure and facilitate the simulation of time-course experiments (44). The activities and permeabilities of individual ion channels and transporters are estimated by least-squares fitting of the model predictions to the experimental data of isolated guinea-pig pancreatic duct (intracellular pH, Cl-, membrane potential, luminal pH and volume).
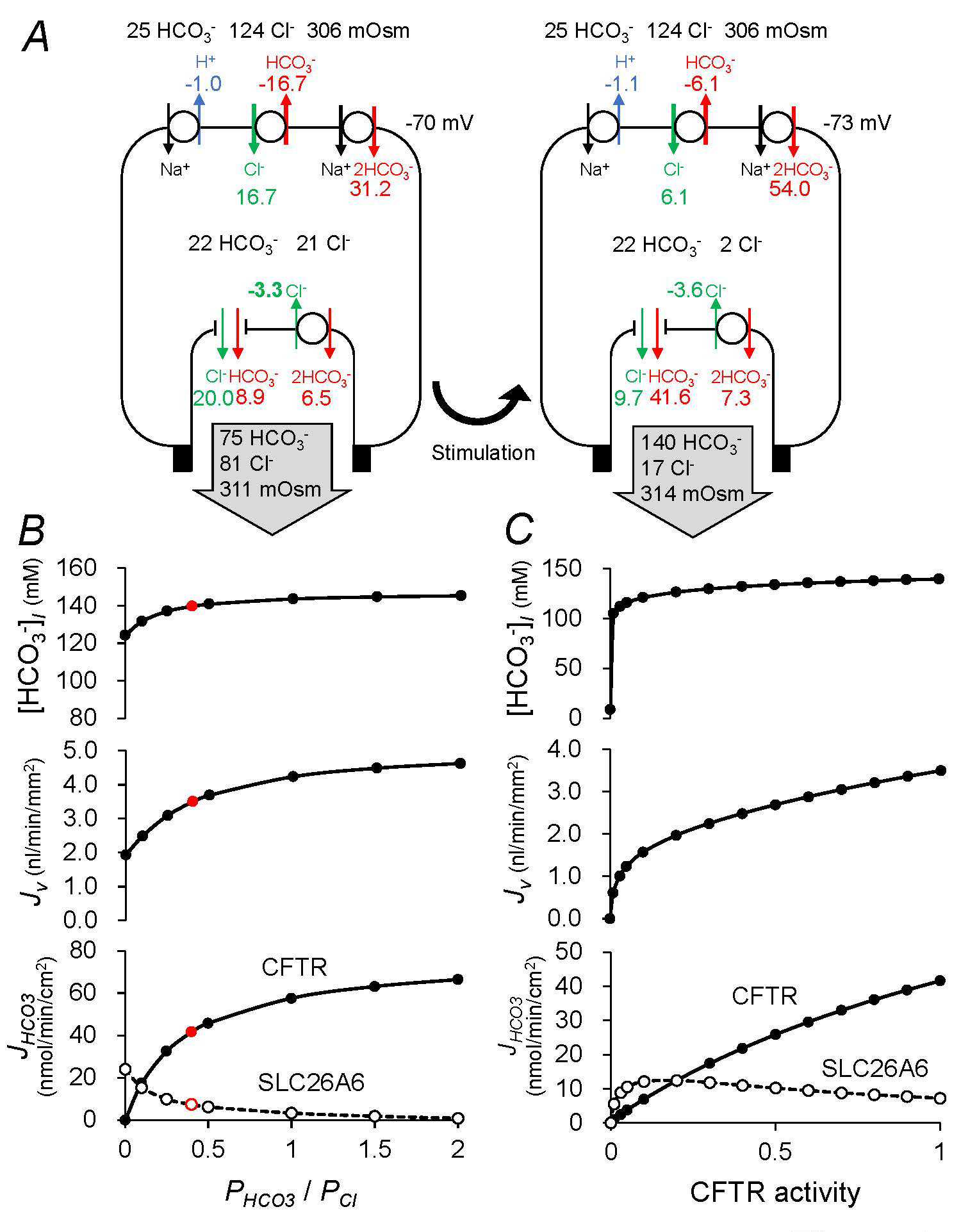
The pancreatic duct epithelium is represented as a 4-compartment system comprising the bath, the lateral intercellular space, the cytoplasm, and the lumen. In the perfused duct model, the composition of the luminal fluid is set to pre-defined values. Alternatively, in the secreting duct model (Figure 7A), the composition of the luminal fluid is allowed to evolve with time and defined by the fluid secreted by the epithelial cells. Stimulation is replicated by increasing the activities of the basolateral Na+-HCO3− cotransporter (NBCe1-B, SLC4A4) and apical Cl−-HCO3− exchanger (SLC26A6), increasing the basolateral K+ permeability and apical Cl− and HCO3− permeabilities (CFTR), and reducing the activity of the basolateral Cl−-HCO3− exchanger (AE2, SLC4A2). Under these conditions, the model secretes ~140 mM HCO3− at a rate of ~3.5 nl min−1 mm−2, which is consistent with experimental observations (15, 45).
Figure 7A shows steady-state fluxes of HCO3−, H+, and Cl- at rest and following cAMP stimulation in the model. Intracellular/luminal/bath Cl−/HCO3− concentrations, osmolarities, and basolateral potential differences are shown as well. In the steady-state, ion compositions of the effluent (pancreatic juice), the luminal fluid, and the fluid secreted by the epithelial cells are identical. While the apical HCO3− flux at rest is divided between CFTR and SLC26A6 (assumed to be 1Cl−:2HCO3− stoichiometry in the standard model), the apical HCO3− flux under cAMP stimulation is predominantly (~90%) via CFTR rather than SLC26A6. The luminal fluid is ~3% hypertonic compared to the bath under stimulation.
The HCO3−/Cl− permeability ratio (PHCO3/PCl) of CFTR has been estimated to be 0.2~0.5 and reported to be regulated by Cl−-sensitive kinases (38). PHCO3/PCl is set at 0.4 in the standard model (Figure 7A). Increasing the ratio to 1.0 has a little effect on the volume (Jv) and HCO3− concentration ([HCO3−]l) of the secreted fluid (Figure 7B). When the ratio is decreased to zero, SLC26A6 compensates for the defective HCO3− transport of by CFTR and the model still secretes ~120 mM HCO3− at a rate of ~2 nl min−1 mm−2.
CFTR Cl− permeability is set at 60 x 10-6 cm s-1 in the standard model. Figure 7C shows the effects of varying CFTR activity on [HCO3−]l, Jv, and apical HCO3− fluxes (JHCO3) via CFTR and SLC26A6. When CFTR activity is completely lost, SLC26A6 by itself cannot support HCO3− and Cl− secretion and there is no fluid secretion. Residual CFTR activity as low as 10% in combination with SLC26A6 accomplishes fluid secretion containing ~120 mM HCO3− at a rate of ~1.5 nl min−1 mm−2. Effective treatment of cystic fibrosis requires restoration of CFTR function and different organs have different requirements for CFTR function. Ion transport in airway epithelium is normalized when 6-10% of cells have corrected CFTR function (5). Recent studies in young children with cystic fibrosis suggest improvement of pancreatic exocrine function by CFTR modulators (34).
Although the computational model is simple and lacks molecular interactions between CFTR and other transporters and regulation by intracellular Cl- (20), it well reproduces the experimental data including the redundancy of acid/base transporters: CFTR and SLC26A6 in the apical membrane and NBC and NHE in the basolateral membrane (44).
Figure 7. Computational model of HCO3- secretion by pancreatic duct cell. (A) A secreting model of guinea-pig pancreatic duct epithelium shows steady-state fluxes of HCO3− (red font), H+ (blue font), and Cl- (green font) mediated by (clockwise from top left) NHE1, AE2, NBC1, SLC26A6 and CFTR. Also shown are the steady-state intracellular/luminal/bath Cl−/HCO3− concentrations, osmolarities, and basolateral potential difference (black font). The left panel represents the unstimulated duct and the right panel shows the effect of altering the transporter/channel activities to represent cAMP stimulation. The Cl−:HCO3− stoichiometry of the SLC26A6 exchanger is assumed to be 1:2. Fluxes are given in nmol min−1 cm−2 (normalized to the luminal surface area of the epithelium) (B) Effects of varying the HCO3−/Cl− permeability ratio of CFTR on HCO3− and fluid secretion in the secreting duct model. Steady-state values in the cAMP-stimulated condition of secreted HCO3− concentration ([HCO3−]l), secretory volume flow (Jv), and net apical HCO3− fluxes via CFTR and SLC26A6 (JHCO3) as a function of the HCO3−/Cl− permeability ratio (PHCO3/PCl) of CFTR. The sum of the permeabilities (PHCO3 + PCl) is maintained at a constant value. (C) Effects of CFTR activity on HCO3− and fluid secretion in the secreting duct model. Steady-state values in the cAMP-stimulated condition of [HCO3−]l, Jv, and JHCO3 via CFTR and SLC26A6 as a function of the CFTR activity. (Adapted from reference (44)).
VII. Acknowledgements
This work was supported by grants from the Japanese Society for the Promotion of Science and the Japanese study group for pediatric rare and intractable hepato-biliary-pancreatic diseases provided by the Ministry of Health, Labor, and Welfare of Japan.
VIII. References
- Argent BE, and Gray MA, Steward, M.C., Case, R.M. Cell Physiology of Pancreatic Ducts. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. Elsevier, 2006, p. 1371-1396.
- Bruce JI, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, and Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem 274: 20561-20568, 1999. PMID: 10400686.
- Busik JV, Hootman SR, Greenidge CA, and Henry DN. Glucose-specific regulation of aldose reductase in capan-1 human pancreatic duct cells In vitro. J Clin Invest 100: 1685-1692, 1997. PMID: 9312166.
- Chan HC, Law SH, Leung PS, Fu LX, and Wong PY. Angiotensin II receptor type I-regulated anion secretion in cystic fibrosis pancreatic duct cells. J Membr Biol 156: 241-249, 1997. PMID: 9096065.
- Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16: 45-56, 2015. PMID: 25404111.
- Czako L, Yamamoto M, and Otsuki M. Pancreatic fluid hypersecretion in rats after acute pancreatitis. Dig Dis Sci 42: 265-272, 1997. PMID: 9052504.
- Futakuchi S, Ishiguro H, Naruse S, Ko SB, Fujiki K, Yamamoto A, Nakakuki M, Song Y, Steward MC, Kondo T, and Goto H. High glucose inhibits HCO3- and fluid secretion in rat pancreatic ducts. Pflugers Arch 459: 215-226, 2009. PMID: PMID: 19756716.
- Hallenbeck GA. Biliary and pancreatic intraductal pressures. In: Handbook of Physiology Alimentary Canal Section, edited by Code CF, Heidal, W. American Physiological Society, 1967, p. 1007-1025.
- Hamada H, Ishiguro H, Yamamoto A, Shimano-Futakuchi S, Ko SB, Yoshikawa T, Goto H, Kitagawa M, Hayakawa T, Seo Y, and Naruse S. Dual effects of n-alcohols on fluid secretion from guinea pig pancreatic ducts. Am J Physiol Cell Physiol 288: C1431-1439, 2005. PMID: 15659715.
- Hayashi M, Inagaki A, Novak I, and Matsuda H. The adenosine A2B receptor is involved in anion secretion in human pancreatic duct Capan-1 epithelial cells. Pflugers Arch 468: 1171-1181, 2016. PMID: 26965147.
- Hegyi P, Rakonczay Z, Jr., Tiszlavicz L, Varro A, Toth A, Racz G, Varga G, Gray MA, and Argent BE. Protein kinase C mediates the inhibitory effect of substance P on HCO3- secretion from guinea pig pancreatic ducts. Am J Physiol Cell Physiol 288: C1030-1041, 2005. PMID: 15625303.
- Ikeura T, Kataoka Y, Wakabayashi T, Mori T, Takamori Y, Takamido S, Okazaki K, and Yamada H. Effects of sensory denervation by neonatal capsaicin administration on experimental pancreatitis induced by dibutyltin dichloride. Med Mol Morphol 40: 141-149, 2007. PMID: 17874046.
- Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, and Steward MC. Luminal ATP stimulates fluid and HCO3- secretion in guinea-pig pancreatic duct. J Physiol 519 (Pt 2): 551-558, 1999. PMID: 10457070.
- Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, and Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol 528 Pt 2: 305-315, 2000. PMID: 11034620.
- Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, and Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 511 (Pt 2): 407-422, 1998. PMID: 9706019.
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, and Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133: 315-326, 2009. PMID: 19204187.
- Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, and Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J Gen Physiol 120: 617-628, 2002. PMID: 19204187.
- Ishiguro H, Steward MC, Wilson RW, and Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol 495 ( Pt 1): 179-191, 1996. PMID: 8866361.
- Ishiguro H, and Yamamoto A, Steward, M.C. Pancreatic Bicarbonate Secretion. In: Encyclopedia of Gastroenterology, edited by Kuipers EJ. Academic Press, 2021, p. 24-29.
- Jun I, and Lee MG, Muallem, S. Molecular mechanisms of pancreatic bicarbonate secretion. Pancreapedia: Exocrine Pancreas Knowledge Base 2020. DOI: 10.3998/panc.2020.06.
- Kim MH, Choi BH, Jung SR, Sernka TJ, Kim S, Kim KT, Hille B, Nguyen TD, and Koh DS. Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. J Biol Chem 283: 18711-18720, 2008. PMID: 18448425.
- Ko SB, Naruse S, Kitagawa M, Ishiguro H, Murakami M, and Hayakawa T. Arginine vasopressin inhibits fluid secretion in guinea pig pancreatic duct cells. Am J Physiol 277: G48-54, 1999. PMID: 10409150.
- Ko SB, Yamamoto A, Azuma S, Song H, Kamimura K, Nakakuki M, Gray MA, Becq F, Ishiguro H, and Goto H. Effects of CFTR gene silencing by siRNA or the luminal application of a CFTR activator on fluid secretion from guinea-pig pancreatic duct cells. Biochem Biophys Res Commun 410: 904-909, 2011. PMID: 21708133.
- Kordas KS, Sperlagh B, Tihanyi T, Topa L, Steward MC, Varga G, and Kittel A. ATP and ATPase secretion by exocrine pancreas in rat, guinea pig, and human. Pancreas 29: 53-60, 2004. PMID: 15211112.
- Korpi ER, Makela R, and Uusi-Oukari M. Ethanol: Novel Actions on Nerve Cell Physiology Explain Impaired Functions. News Physiol Sci 13: 164-170, 1998. PMID: 11390783.
- Kulaksiz H, Schmid A, Honscheid M, Eissele R, Klempnauer J, and Cetin Y. Guanylin in the human pancreas: a novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system. Histochem Cell Biol 115: 131-145, 2001. PMID: 11444148.
- Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, Nakazawa T, Ohara H, Nomura T, Joh T, Shirai T, and Itoh M. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology 124: 1010-1019, 2003. PMID: 12671898.
- Larusch J, and Whitcomb DC. Genetics of pancreatitis with a focus on the pancreatic ducts. Minerva Gastroenterol Dietol 58: 299-308, 2012. PMID: 23207607.
- Laukkarinen JM, Weiss ER, van Acker GJ, Steer ML, and Perides G. Protease-activated receptor-2 exerts contrasting model-specific effects on acute experimental pancreatitis. J Biol Chem 283: 20703-20712, 2008. PMID: 18511423.
- Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 580: 31-37, 2007. PMID: 17218353.
- Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y, and Research Committee on Intractable Pancreatic D. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. Am J Gastroenterol 96: 2622-2627, 2001. PMID: 11569685.
- Luo X, Zheng W, Yan M, Lee MG, and Muallem S. Multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells. Am J Physiol 277: C205-215, 1999. PMID: 10444396.
- Maleth J, Balazs A, Pallagi P, Balla Z, Kui B, Katona M, Judak L, Nemeth I, Kemeny LV, Rakonczay Z, Jr., Venglovecz V, Foldesi I, Peto Z, Somoracz A, Borka K, Perdomo D, Lukacs GL, Gray MA, Monterisi S, Zaccolo M, Sendler M, Mayerle J, Kuhn JP, Lerch MM, Sahin-Toth M, and Hegyi P. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 148: 427-439 e416, 2015. PMID: 25447846.
- Megalaa R, Gopalareddy V, Champion E, and Goralski JL. Time for a gut check: Pancreatic sufficiency resulting from CFTR modulator use. Pediatr Pulmonol 54: E16-E18, 2019. PMID: 31066218.
- Namkung W, Lee JA, Ahn W, Han W, Kwon SW, Ahn DS, Kim KH, and Lee MG. Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl- -dependent HCO3 transport in pancreatic duct cells. J Biol Chem 278: 200-207, 2003. PMID: 12409301.
- Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal 4: 237-253, 2008. PMID: 18368520.
- Pallagi P, Venglovecz V, Rakonczay Z, Jr., Borka K, Korompay A, Ozsvari B, Judak L, Sahin-Toth M, Geisz A, Schnur A, Maleth J, Takacs T, Gray MA, Argent BE, Mayerle J, Lerch MM, Wittmann T, and Hegyi P. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR CL- channels and luminal anion exchangers. Gastroenterology 141: 2228-2239 e2226, 2011. PMID: 21893120.
- Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, and Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620-631, 2010. PMID: 20398666.
- Sohma Y, Gray MA, Imai Y, and Argent BE. HCO3- transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol 176: 77-100, 2000. PMID: 10882430.
- Sorensen CE, and Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276: 32925-32932, 2001. PMID: 11387334.
- Suzuki A, Naruse S, Kitagawa M, Ishiguro H, Yoshikawa T, Ko SB, Yamamoto A, Hamada H, and Hayakawa T. 5-hydroxytryptamine strongly inhibits fluid secretion in guinea pig pancreatic duct cells. J Clin Invest 108: 749-756, 2001. PMID: 11544281.
- Szalmay G, Varga G, Kajiyama F, Yang XS, Lang TF, Case RM, and Steward MC. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol 535: 795-807, 2001. PMID: 11559776.
- Whitcomb DC, and Ermentrout GB. A mathematical model of the pancreatic duct cell generating high bicarbonate concentrations in pancreatic juice. Pancreas 29: e30-40, 2004. PMID: 15257112.
- Yamaguchi M, Steward MC, Smallbone K, Sohma Y, Yamamoto A, Ko SB, Kondo T, and Ishiguro H. Bicarbonate-rich fluid secretion predicted by a computational model of guinea-pig pancreatic duct epithelium. J Physiol 595: 1947-1972, 2017. PMID: 27995646.
- Yamamoto A, Ishiguro H, Ko SB, Suzuki A, Wang Y, Hamada H, Mizuno N, Kitagawa M, Hayakawa T, and Naruse S. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol 551: 917-926, 2003. PMID: 12847207.