Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2021.03
I. INTRODUCTION
The exocrine pancreas produces and secretes multiple digestive enzymes and has been the model in which the structure and functional organization of the mammalian secretory pathway was originally discovered and intensively studied (59, 83) The pancreatic acinar cells exhibit one of the highest protein synthesis rates among mammalian cells. More than 90% of the newly synthesized proteins are targeted to the secretory pathway (71) and packaged into large secretory granules, called zymogen granules (ZG). In contrast to the smaller neuroendocrine and endocrine granules, ZGs have an averaged diameter of around 1µm. They are responsible for transport, storage and secretion of digestive enzymes and have long been a model for studying the general mechanisms of secretory granule biogenesis and regulated exocytosis. Stimulation of the acinar cells by secretagogues such as acetylcholine and cholecystokinin triggers fusion of ZG membrane with the apical plasma membrane and the release of digestive enzymes into the pancreatic ductal system. In the duodenum, trypsinogen is converted to trypsin by proteolytic cleavage via enterokinase and activated trypsin then proteolytically activates the other zymogen enzymes (9, 58).
Physiological stimulation of acinar cells by secretagogues triggers local apical Ca2+ spiking, fusion of ZG membrane with the apical membrane, and exocytosis (53, 60, 61, 85). In contrast to the physiological condition, supramaximal CCK stimulation elicits sustained elevation of cytosolic [Ca2+] and leads to mistrafficking of digestive and lysosomal enzymes, inhibition of apical secretion and abnormal exocytosis redirected to basolateral plasma membrane all of which are believed to contribute to the pathogenesis of acute pancreatitis (23, 68). The ZG content contains the digestive enzymes and associated proteins which are the major protein components of the pancreatic juice secreted into the duodenum. The ZG membrane carries at least part of the molecular machinery responsible for digestive enzyme sorting, granule trafficking and exocytosis. For example, digestive enzyme sorting and packaging will, at least to some extent, depend on interactions between ZG content and ZG membrane components exposed to the lumen of ZGs. The cytoplasmic surface of ZG membrane must contain vesicular trafficking proteins including Rabs, SNARE proteins as well as molecular motors to interact with cytoskeleton. Defective ZG biogenesis and trafficking can result in various pancreatic diseases such as acute and chronic pancreatitis (23, 31, 68). A comprehensive understanding of the protein composition of the ZG content and membrane is necessary to provide critical insights in the biogenesis and regulated secretion of pancreatic ZGs.
II. ZG PROTEIN COMPOSITION
A. Zymogens and digestive enzymes
The major secretory products of the acinar cells, namely the content of ZGs, are digestive enzymes which belong to five functional groups of hydrolytic enzymes including endo- and exo-proteases, lipases, glycosidases, and nucleases. In contrast to endocrine cells which often produces a predominant peptide or protein product such as insulin, acinar cells synthesize, package and secrete a mixture of nearly 20 different enzymes and isoenzymes including amylase, trypsinogens, chymotrypsinogens, carboxypeptidases, esterases, lipases and ribonucleases. Most pancreatic proteases are synthesized as inactive precursors or zymogens which only become activated by a cascade of limited proteolysis within the intestinal lumen. Because of the importance of ZG to digestive enzyme storage and regulated secretion and as a general model for secretory vesicles, the identification and characterization of both the soluble and membrane proteins of ZGs have been of great interest in the field. In an early pioneering study, the secreted ZG contents from the guinea pig exocrine pancreas were analyzed by two-dimensional gel electrophoresis which resolved 19 distinct high molecular weight proteins. Thirteen of the 19 proteins were identified by actual or potential enzymatic activity (69). In more recent studies using mass spectrometry-based proteomics analyses, the identities of these enzymes have been confirmed and additional isoforms were found (12, 63). The ZG contents make up the major protein components of the pancreatic juice secreted into the duodenum. Therefore, the identification of ZG content proteins also has a significant impact on biomarker studies in the pancreatic juice (15).
B. Components and topology of the ZG membrane proteins
It is believed that the integral and peripheral ZG membrane proteins serve critical functions for zymogen sorting/packaging, vesicular trafficking and regulated exocytosis. Therefore, a comprehensive identification of ZG membrane proteins is expected to shed new lights on our understanding of ZG biogenesis and secretion. In early studies (20, 37, 52), characterization of rat ZG membranes by SDS-PAGE indicated a relatively simple protein composition of about 10 components with GP2 (glycoprotein 2) accounting for 40% of the proteins. In the past decade, studies have been carried out to characterize ZG membrane proteins using two-dimensional gel electrophoresis. These efforts led to the identifications of two additional major ZG membrane components, GP3 (glycoprotein 3) (79) and membrane dipeptidase (35), by N-terminal amino acid sequence analysis. However, due to the lack of sensitive tools for protein identification, the identities of many spots resolved on the 2D gels remained unknown. In another study, fourteen spots corresponding to small GTP binding proteins were resolved on a 2D gel of ZG membrane proteins by [35S]GTPγS blotting analysis (28). However, the identities of the spots were not determined. Different from the above abundant ZG membrane proteins, a number of low abundance regulatory proteins have been identified on ZG membrane by immunoblotting and immunocytochemistry. Examples of these proteins included the small GTPase, Rab3D (55, 77) and the SNARE proteins, VAMP2 (vesicle associated membrane protein 2) (25) and syntaxin 3 (22, 34). More recently, VAMP 8 was found on ZG membrane and to play a major physiological role in regulated exocytosis (81). Despite the significant amount of knowledge of ZG membrane proteins accumulated in the past decades on an individual basis, a comprehensive characterization of the membrane protein components of this organelle was not achieved until the application of modern mass spectrometry revealed a much more complex makeup of the ZG membrane (11, 12, 63).
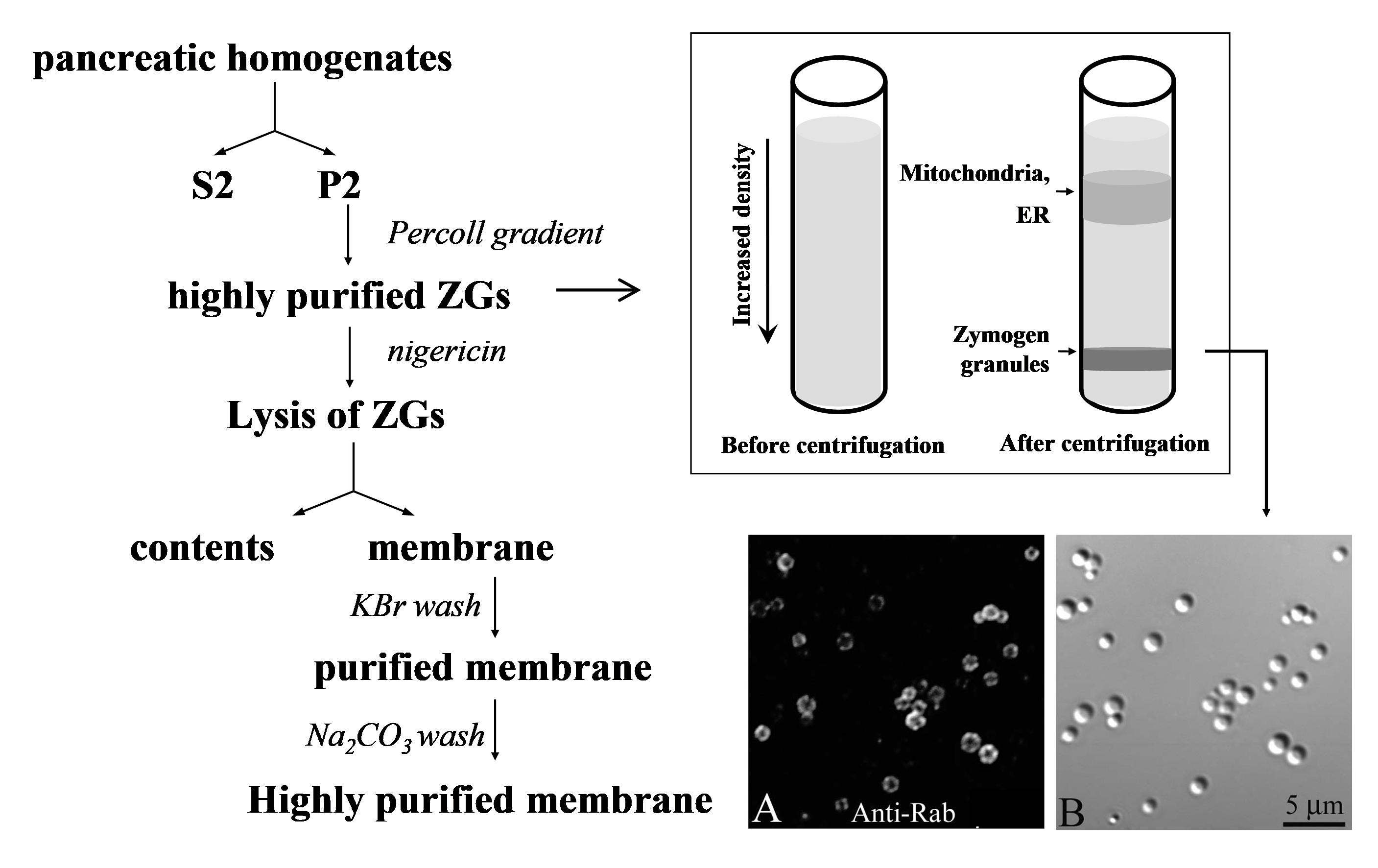
Organellar proteomics represents an analytical strategy that combines biochemical fractionation and comprehensive protein identification. Initial purification of organelles leads to reduced sample complexity and links proteomics data to functional analysis (7, 80, 90). In the past decade, organellar proteomic analysis has been carried out for virtually every subcellular compartment in the mammalian secretory pathway including Golgi, ER and secretory granules (80). The first comprehensive analysis of rat ZG membrane were carried out by combining modern mass spectrometry-based proteomics technologies and a well-established protocol of ZG purification (12). Using this protocol (outlined in Figure 1,left), a crude granule pellet (P2) was prepared by two consecutive low speed centrifugations and then further purified by an ultracentrifugation in a self-forming Percoll gradient. A heavy white band, containing highly purified ZGs, was observed and collected just above the bottom of the tube. The ZG membrane and content proteins were then separated by osmotic lysis of ZGs with the ionophore, nigericin, followed by ultracentrifugation. The membrane pellet was washed first with 0.25M KBr and then with 0.1M Na2CO3 (pH 11.0) to remove soluble content proteins and loosely associated proteins. The known ZG membrane marker such as Rab3D was highly enriched in the purified membrane fractions (Figure 1, right).
Figure 1. Outline of ZG membrane purification. Left: Rat pancreata were homogenized and then centrifuged in two consecutive low speed steps to generate a crudeparticulate fraction (P2) enriched in ZGs. The particulate was resuspended, mixed with equal volume of Percoll, and ultracentrifuged. The dense white ZG band was then collected and washed. To purify ZG membrane, the isolated ZGs were lysed with nigericin and ultracentrifuged to separate contents and membranes. The membrane pellet was then washed sequentially with 250 mM KBr and 0.1 M Na2CO3 (pH 11.0). Right: Top shows a cartoon to illustrate the Percoll gradient ultracentrifugation; at the bottom are Nomarski and fluorescent images of purified ZGs to demonstrate the purity of ZGs and the positive staining of a ZG marker, Rab3D. (Reproduced from reference (12)).
These results indicated a much more complex protein composition of the ZG membrane. By combining multiple separation strategies including one-, two-dimensional gel electrophoresis and two-dimensional HPLC with tandem mass spectrometry, over 100 proteins were identified from purified ZG membrane (12). Most of the known ZG membrane proteins were identified, including high abundance matrix proteins such as GP2, GP3, ZG16 and syncollin which are likely involved in ZG sorting and packaging, and low abundance proteins such as dynactin2 (48) and VAMP2 which are involved in ZG trafficking and exocytosis. A large number of novel ZG membrane proteins were also identified, including the SNARE protein, SNAP 29, the small G proteins Rab27B, Rab11A and Rap1 and the molecular motor protein, myosin Vc. Indicative of the interest in understanding the ZG membrane proteome, a later study (63) using 1D SDS-PAGE coupled with 1D LC-MS/MS identified, in a more redundant database, 371 proteins from both ZG membrane and content. A large degree of overlap between proteins was found in these two independent studies. The overlap is higher (nearly 100%) for high score protein identifications and major new observations including all the new vesicular trafficking proteins, whereas the disagreement increases in low score identifications some of which may be contaminants. Representative ZG proteins found in multiple proteomic analyses (11, 12, 63) as well as in some individual studies are summarized in Table 1.
Table 1.
Protein name
NCBI #
MW
pI
Ref
Digestive enzymes
Alpha-amylase
62644218
51020
8.42
Anionic trypsin precursor
67548
28363
4.69
Chymotrypsin C
1705913
30919
5.64
Carboxypeptidase A1 precursor
8393183
50282
5.38
Carboxypeptidase A2 precursor
61556903
50269
5.17
Cationic trypsinogen
27465583
28821
7.45
Chymotrypsin B
6978717
25934
4.90
Colipase
203503
13597
8.04
Elastase 2
6978803
27274
8.81
Pancreatic lipase
1865644
54494
6.6
Pancreatic lipase related protein 1
14091772
57122
5.79
Similar to elastase 3B
62649890
30806
5.47
Sterol esterase
1083805
72537
5.37
Small GTPases
Rab11A
2463536
24509
5.98
(29, 38)
Rab14
420272
24078
5.85
Rab1A
56605816
25670
5.95
RAB27B
16758202
27382
5.38
(10)
RAB3D
18034781
26332
4.75
(55, 77)
Rab8A
77748034
23668
9.15
(17)
Rap1
52138628
21201
5.37
(66)
ZG matrix proteins
Clusterin
46048420
56070
5.53
(54)
GP2
121538
62355
4.9
(20, 62)
GP3
17105374
58695
6.03
(79, 87)
Syncollin
20806121
17780
8.61
(1, 16)
ZG16
19705541
17316
9.79
(47)
Transporters, pumps and ion channels
Cation-chloride cotransporter 6
13516403
95862
8.09
Cation-chloride cotransporter 9
23495276
77073
6.2
Chloride channel protein 3
4762023
90855
5.88
(45)
L-type amino acid transporter 1
12643400
55903
8.18
Vacuolar-type H+-ATPase 115 kDa subunit, a1 isoform
13928826
102385
6.04
(66)
Vesicular trafficking proteins
Cysteine string protein
1095322
24892
4.93
(8)
Dynactin 2
50926127
44148
5.14
(48)
Myosin Vc
62653910
228341
8.17
SCAMP1a
158749626
37999
7.61
SNAP29
7769720
29000
5.40
Synaptotagmin-like protein 1
71043698
59471
5.53
Synaptotagmin-like protein 4
17939356
75900
9.08
Syntaxin 7b
55741787
29851
5.32
Syntaxin 12
77695930
31187
5.23
VAMP 2
51704188
12691
7.84
(24)
VAMP 8
13929182
12512
8.93
Other proteins
CD47 antigen
55250722
32995
8.91
CD59 antigen
6978635
13790
8.9
CD63 antigen
38648866
29617
7.37
Dipeptidase 1
16758372
48023
5.68
(36)
Ectonucleoside triphosphate diphosphohydrolase 1 (CD39)
12018242
57337
7.47
Gamma-glutamyl transpeptidase
16758696
66667
8.46
(6)
Itmap1
5916203
72874
6.07
(41)
Polymeric immunoglobulin
receptor27151742
84798
5.07
Table 1. Representative pancreatic ZG proteins identified from proteomics analysis and their major functional categories. Representative ZG proteins from major functional categories are listed. These proteins were identified on purified rat pancreatic ZGs from multiple proteomics studies (11, 12, 63). Proteins potentially co-purified from other subcellular organelles are not included. References for their original discovery or with independent functional characterization or immunostaining are also included. Note: a) SCAMP2, 3, 4, and, b) syntaxin 3 were also identified on ZG membrane.
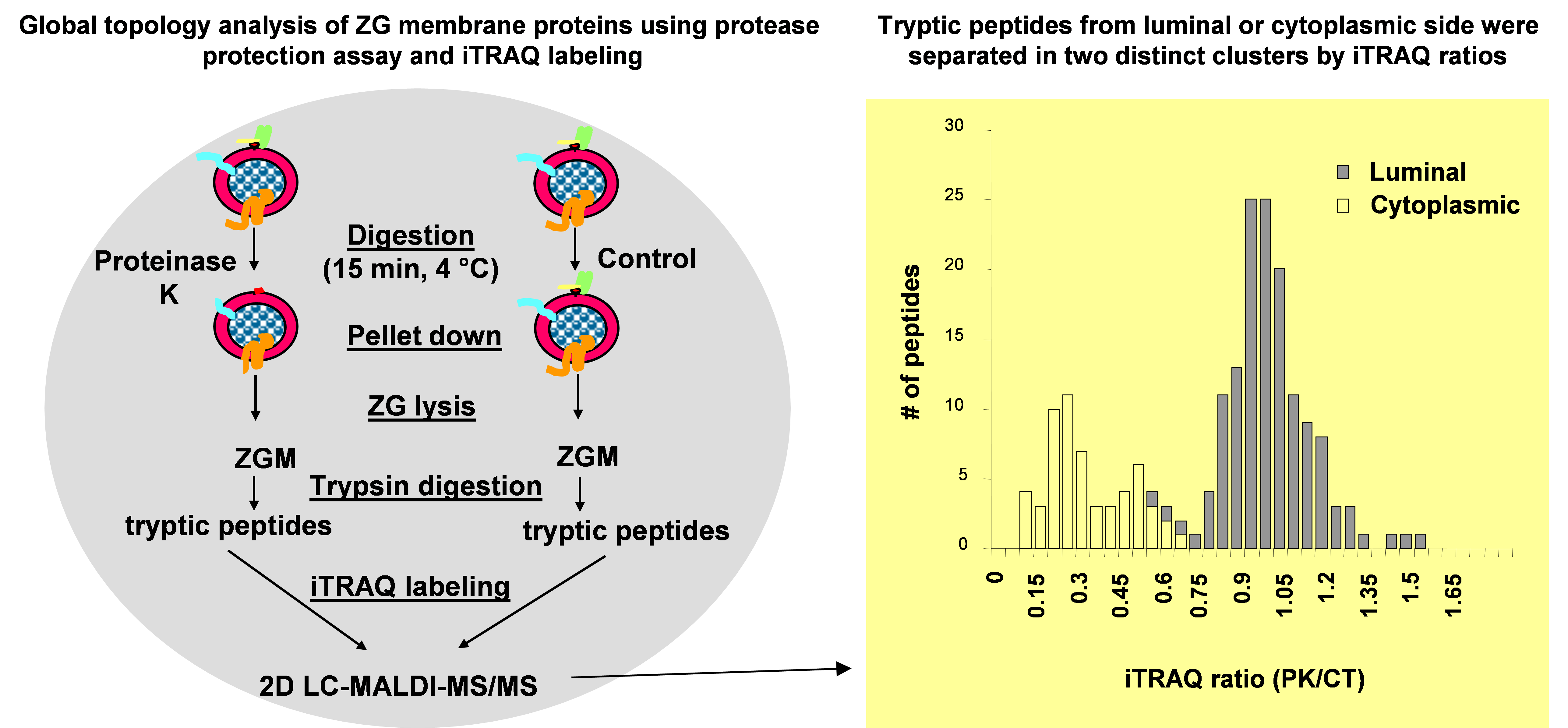
As a second step towards a comprehensive architectural model of ZG membrane, a systematic topology analysis of ZG membrane proteins was performed by combining a global protease protection assay with iTRAQ-based quantitative proteomic technology (11). In this study, isolated ZGs were incubated with or without proteinase K. The control and proteinase K treated ZG membrane proteins were digested with trypsin and the resulting peptides were labeled with iTRAQ reagents to compare the relative abundance of each peptide in the two samples. Because proteinase K treatment removed cytoplasm-sided ZGM proteins or domains, the iTRAQ ratios (proteinase K treated vs. control) from all peptides fell into two clusters. The peptides from cytoplasm-sided proteins or domains showed reduced iTRAQ ratios (<<1.0) whereas those luminal ZG proteins showed little change (ratios around 1.0) (Figure 2). A threshold was determined using a training set of ZG membrane proteins with well-characterized membrane topology. The category with iTRAQ ratios below the threshold included the cytoplasm-sided peripheral membrane proteins, such as synaptotagmin-like protein 1 and Myosin Vc, and membrane proteins with single transmembrane structure or post-translational lipid modification, such as VAMP 2, 8, Syntaxin 7 and all the Rab proteins. The second category with the ratios above the threshold included the lumen-sided peripheral membrane proteins, such as GP2, GP3, syncollin and all of the digestive enzymes. Altogether, this analysis was able to successfully assign the membrane topology for 199 identified ZG proteins (11). The advantage of this technique was to analyze a large number of endogenous proteins simultaneously without the necessity to exogenously express fusion proteins. In addition, this technique was also able to map both cytoplasm- and lumen-sided domains from the same transmembrane proteins.
Figure. 2. Work flow of iTRAQ-based topology analysis of pancreatic ZG membrane proteins. Left: Isolated ZGs were treated with or without proteinase K and then lysed. ZG membrane proteins were digested with trypsin, and resulting peptides were labeled with iTRAQ reagents. The peptides were mixed and analyzed by 2D LC-MALDIMS/MS. Right: iTRAQ ratio distributions of tryptic peptides from ZGM proteins with known topology.A histogram of iTRAQ ratios (proteinase K versus control) from identified peptides illustrates the presence of two distinct clusters of tryptic peptides from cytoplasm-orientated and lumen-orientated ZG proteins, respectively. (Modified from reference (11)).
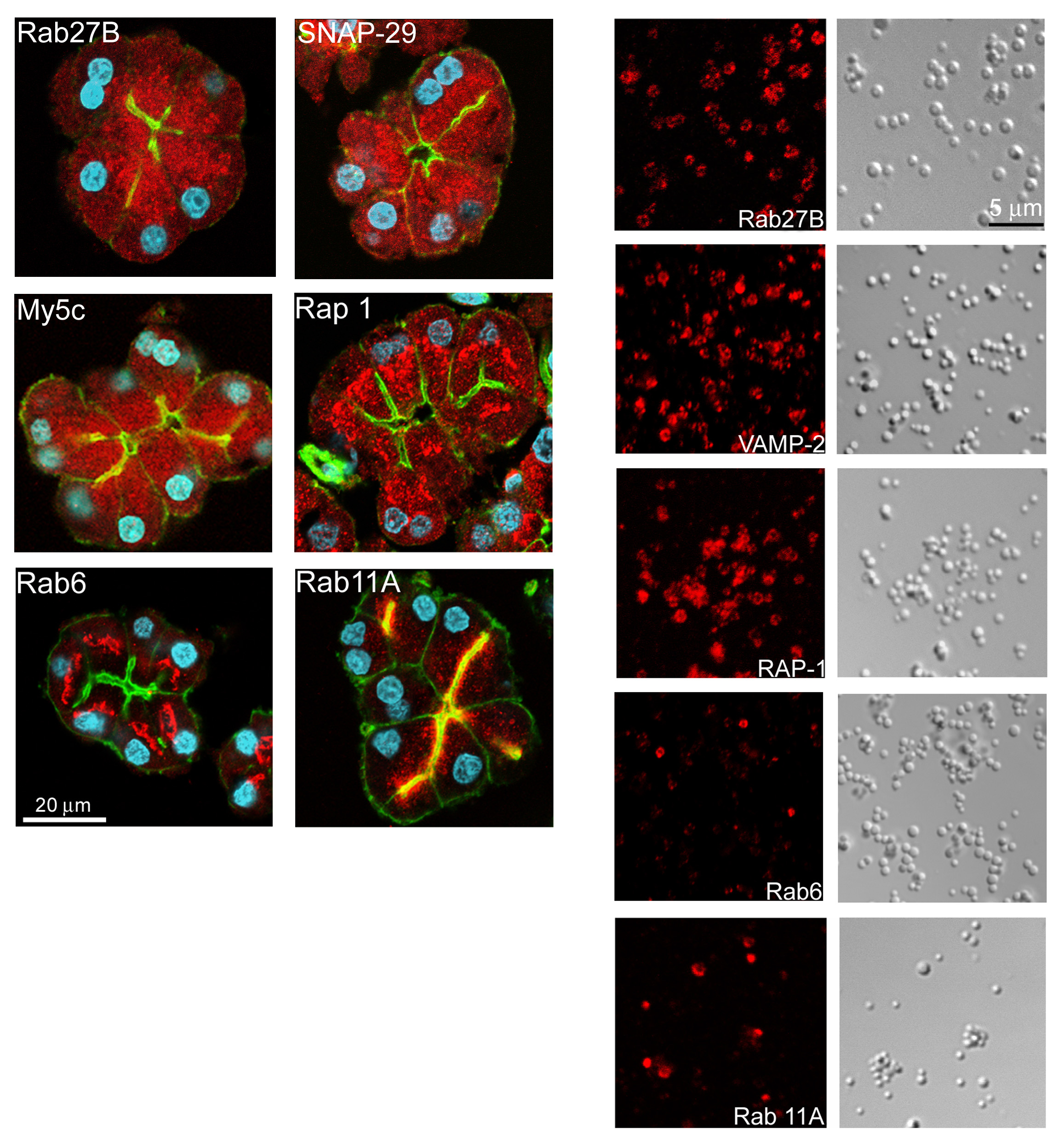
The overall goal of these recent proteomics studies is to build a quantitative, architectural model of the pancreatic ZG which will lead to new hypotheses for subsequent functional analysis of this prototypic secretory granule. The ZG localization of a number of novel proteins, including Rap1, Rab6, Rab11A, Rab27B, SNAP29 and myosin Vc, were confirmed by immunocytochemistry (Figure 3). Several such novel observations have already led to hypothesis-driven functional studies. One example was Rab27B, subsequently demonstrated as an important regulator of acinar exocytosis (10). Other examples included Rap1 (67) and SNAP 29 (82). The fact that Myosin Vc was also identified on ZG membrane in this study led us to hypothesize that Myosin Vc and Rab27B form a complex to tether ZGs at the apical actin web in an analogy to the Rab27A/melanophilin/Myosin Va complex on melanosomes (19, 33, 73, 88, 89). This hypothesis is further supported by the more recent findings of two potential Rab27 effector proteins, synaptotagmin-like protein (Slp) 1 and 4 (11, 63).
Figure 3. Immunolocalization of novel small GTPases and SNARE proteins to isolated ZGs. The ZG localizations of some of the novel small GTPases and SNARE proteins were confirmed at isolated ZGs level by immunocytochemistry and confocal microscopy. The immunofluorescent images (red) together with corresponding DIC images for VAMP2, Rab11a, Rap1 and Rab27b are shown. While VAMP2, Rap1 and Rab27b stained every ZGs as indicated by circles outlining individual ZGs, only a portion of ZGs showed positive Rab11a staining. (Reproduced from reference (12)).
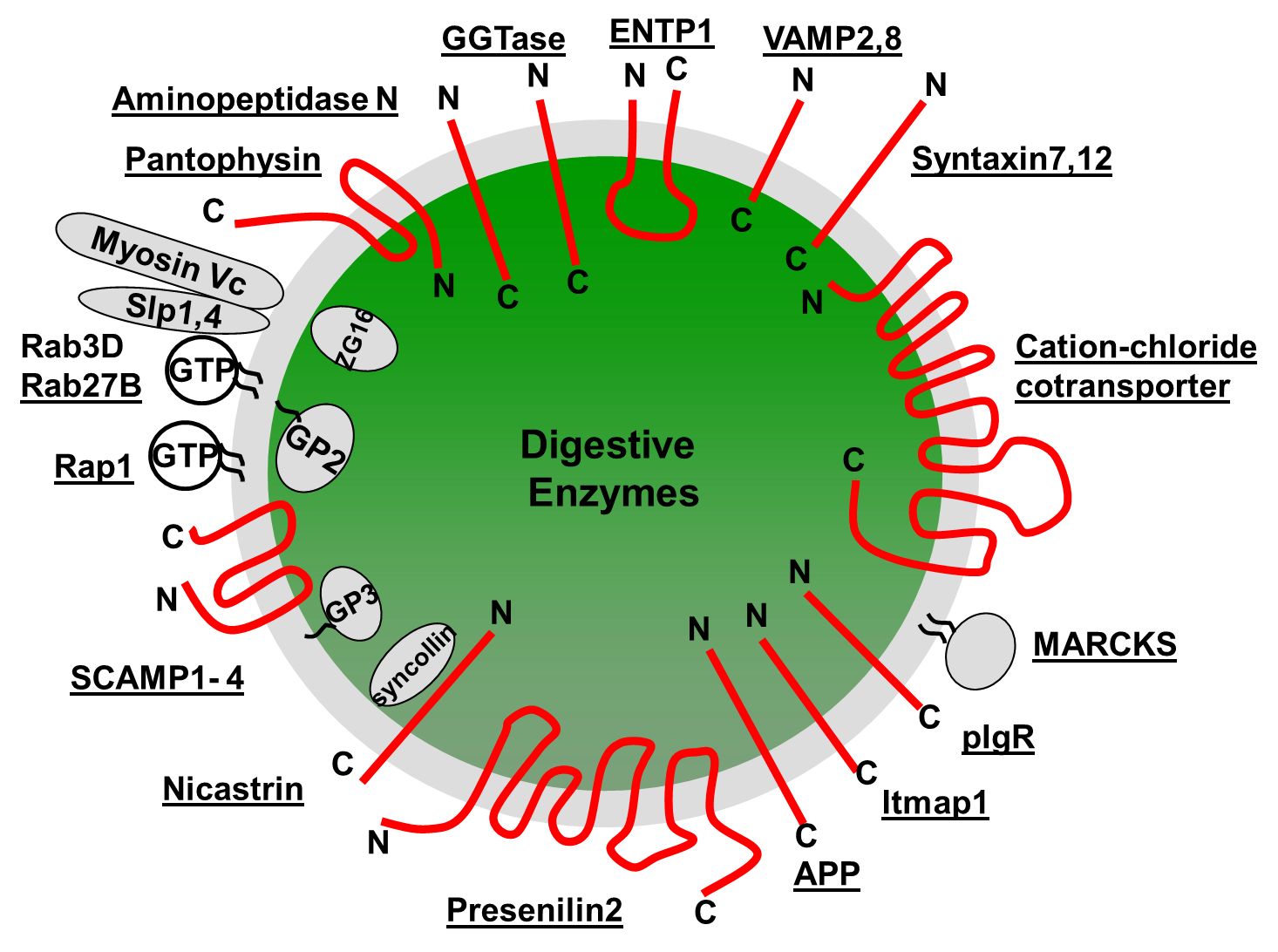
A comprehensive model of the ZG requires the absolute quantity of each individual ZG protein as well as the stoichiometry among different ZG proteins. However, to date, this information has not yet been determined for any ZG protein. Recently, an absolute quantification (AQUA) proteomics strategy (26, 46) using LC-SRM and isotope-labeled synthetic peptides was used to obtain absolute molar abundances for selected mouse ZG proteins, Rab3D and VAMP8 (43). The absolute quantities of mouse Rab3D and VAMP8 were determined as 1242 ± 218 and 2039 ± 151 (Mean ± SEM) copies per ZG. The size distribution and the averaged diameter of ZGs (~750 nm) were determined by atomic force microscopy (43). For an average sized ZG, the densities of these two proteins, if evenly distributed on the ZG membrane, were 702 molecules/µm2 for Rab3D and 1152 molecules/ µm2 for VAMP8, respectively. As a comparison, it was estimated that Rab3A had 10 copies, and VAMP2 70 copies on a synaptic vesicle (an average diameter of 45.18 nm) with their corresponding membrane densities being 1572 and 11,003 molecules/µm2, respectively (74). It is worth noting that some ZG proteins can be present on a subpopulation of ZGs or concentrated on specific domains of the ZG membrane. The average copy numbers and membrane densities determined here can serve as a starting point to further examine the uneven distributions of specific ZG proteins. In addition to mouse and rat ZGs, ZGs were purified from human acini obtained from pancreatic islet transplantation center and the comprehensive constituents of human ZGs were characterized for the first time (43). 180 human ZG proteins were identified including both the membrane and the content proteins. The identification of human ZG specific content and membrane proteins is expected to have a significant impact on translational studies to look for biomarker in pancreatic juice from cancer and pancreatitis patients. From these proteomics analyses in the past decades, a comprehensive molecular model of ZG has emerged to include the protein components, membrane topologies, copy numbers per ZG, and protein-protein interactions. A diagram of such a ZG molecular model with selective ZG membrane proteins is shown in Figure 4.
Figure 4. Molecular architecture of pancreatic zymogen granule proteins. A number of identified ZGM proteins and their topology assignments are shown on a single ZG.
C. Functional categories of ZG proteins
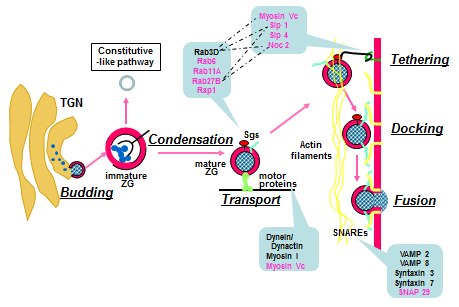
The secretory granules in neuroendocrine, endocrine and exocrine cells share fundamental molecular mechanisms in granule formation, intracellular trafficking and regulated exocytosis (4, 32, 72). For ZGs, this multi-step process includes budding of immature granules off the trans-Golgi network, granule maturation and granule transport towards the apical pole in the vicinity of plasma membrane, tethering/docking at the plasma membrane and regulated exocytosis of ZG contents triggered by the rise of local Ca2+ concentration upon hormonal and neuronal stimulations (Figure 5). In spite of a general model outlined above, the detailed molecular mechanisms are not yet completely understood. The ZG membrane is believed to carry at least part of the molecular machinery responsible for each of these steps. Therefore, the recent comprehensive identification of the ZG protein components has shed new light on ZG biogenesis, trafficking and exocytosis (11, 12, 30, 63). The identified ZG proteins fall into several broad functional categories which link their identities to potential functional importance. These categories include proton pumps and ion channels, enzymes, vesicular trafficking proteins, matrix and glycoproteins, small GTP-binding proteins (Table 1).
Figure 5. A working model of ZG biogenesis and regulated exocytosis. For ZGs, this multi-step process includes the budding of immature granules from the trans-Golgi network (TGN), granule maturation through condensation and transport towards the apical pole in the vicinity of plasma membrane, tethering/docking at the plasma membrane and regulated exocytosis of ZG contents through membrane fusion. (Modified from reference (84)).
The ZG matrix protein group includes the highly abundant ZG proteins such as GP2, GP3, ZG16 and syncollin, previously known to be present on the inner surface of ZG membrane and likely involved in zymogen sorting and packaging (more detailed discussion in the next section) (44, 47, 49, 72). In addition to the high abundance structural proteins and enzymes, many of the known functional and regulatory proteins were also identified on the outer surface of the ZGs. These included vesicular trafficking proteins such as SNARE proteins VAMP 2 and VAMP 8, molecular motors myosin Vc and dynactin 2, a subunit of dynactin adaptor complex for the minus end-driven microtubular transport motor, dynein. A significant number of small GTPases were identified including multiple Rabs and Rap1. Among them, only Rab3D was previously reported on ZGs (55, 56, 76, 77). These newly identified ZG-localized small GTPases represented one of the major novel findings from the extensive proteomics analyses of ZG membrane. Ion channels and transporters are usually low abundant proteins and with multi-transmembrane domains which make them hard to detect by proteomics approaches. Within this category, various subunits of the vacuolar H+-ATPase (V-ATPase) have been constantly identified in multiple proteomics studies (11, 12, 63). A few other ion channels and transporters have also been identified in individual proteomics studies (Table 1). In addition, functional evidence indicates the presence of several ion channel and transporter proteins in ZG membranes (aquaporins, vacuolar-type H+-ATPase, zinc influx transporter SLC30A2). The evidence for the K+ channels Kv7.1 and Kir6.1, for ClC Cl- channels and the vesicular nucleotide transporter SLC17A9 in ZG is less strong. Detailed discussion can be found in the “Channels and Transporters in Zymogen Granule Membranes and their Role in Granule Function: Recent Progress and a Critical Assessment” section by Dr. Frank Thévenod (75).
III. ZG BIOGENESIS - SORTING AND MATURATION
Protein sorting at the trans-Golgi Network (TGN) is particularly important for professional secretory cells, such as pancreatic acinar cells. In contrast to most eukaryotic cells that secrete proteins constitutively via TGN-derived vesicles, acinar cells store their regulated secretory product in granules that undergo exocytosis in a stimulus-dependent manner. In acinar cells, a mixture of different zymogens is packaged within the TGN, some zymogens form protein complexes and progressively aggregate in a Ca2+- and pH- dependent manner. Parts of the Golgi cisternae become dilated and these condensing vacuoles (CV) pinch off to become immature granules (IG) and then mature into ZGs. The selective aggregation of pancreatic secretory proteins has been well documented (14, 18, 51), but the underlying molecular mechanism by which the secretory proteins are sorted into the regulated secretory pathway is still not well understood. Two different hypotheses, not necessarily mutually exclusive, have been developed to explain the selection of content proteins for storage in the secretory granules (4, 32).
The sorting-for-entry hypothesis proposes that the TGN acts as the primary protein sorting station in the biosynthetic transport pathway. This is based on the paradigm for newly synthesized lysosomal hydrolases which are sorted into clathrin-coated vesicles by mannose-6-phosphate receptor (MPR) and then targeted to endosomal membranes. For the regulated secretory proteins, the sorting-for-entry model postulated the presence of TGN membrane associated sorting receptors to facilitate cargo entry into IG. Through this mechanism, only selected secretory proteins can enter IGs, whereas other proteins such as those targeting to the constitutive secretory pathway are efficiently excluded. Careful immuno-electron microscopy observations have demonstrated the segregation of regulated and constitutive cargo at the level of the TGN (57), supporting an active sorting mechanism.
In the sorting-by-retention hypothesis, the IG (immature granules) serves as an important post-TGN sorting station. In this model, protein entrance into IGs is largely unselective and high-order intermolecular associations allow regulated secretory proteins for efficient retention within maturing granules. Concurrently, a subset of protein components is removed via receptor-mediated sorting or bulk flow. The driving force underlying this subtractive retention involves assembly of granule core proteins within IGs by aggregation/condensation, the progressive protein insolubility within the luminal environment of maturing granules in a Ca2+- and pH- dependent manner. Condensation of regulated secretory proteins allow them to remain in the maturing granules while the lysosomal proteins are removed by constitutive-like vesicle budding.
In pancreatic acinar cells, membrane proteins involved in the sorting and packaging of zymogens are expected to have their functional domains exposed on the luminal side of the ZG membrane. Studies have been focused on several abundant luminal ZG membrane proteins for their potential roles in zymogen sorting and ZG formation (72). GP2 represents up to 40% of the total ZG membrane proteins in rat ZG (65). The membrane association of GP2 is via a glycosylphosphatidylinositol (GPI) anchor (50, 70). Since GP2 can form stable complexes with zymogens at mildly acidic pH but not at alkaline pH (13, 50), it was suggested that GP2 may act as a sortase for aggregated secretory proteins (42). However, the findings that ZGs in mouse pancreas can form in the absence of GP2 indicated it is not required for ZG biogenesis (91). Another abundant luminal ZG membrane protein, syncollin, is a component of lipid rafts (44). While the rates of synthesis and intracellular transport of secretory proteins were reduced in syncollin-deficient mice, these mice are viable and showed no detectable changes in pancreatic morphology, regulated exocytosis, or zymogen content (2). So syncollin does not seem to be required for ZG biogenesis either. While several other luminal membrane-associated ZG proteins have also been studied in ZG biogenesis, it is still not clear that any of these proteins alone is indispensable for ZG formation. However, it has been shown that the assembly of a proteoglycan scaffold at the ZG membrane is supporting efficient packaging of zymogens and the proper formation of stimulus-competent storage granules in acinar cells of the pancreas (3). Other proteins identified as associated with the inner surface of the ZGM include chymase and peptidyl-prolyl isomerase B (7).
IV. ZG TRAFFICKING AND EXOCYTOSIS
Upon nervous and/or hormonal stimulation (86), ZGs move towards the apical plasma membrane in a microtubule- and actin-dependent manner (48, 78). Secretagogue stimulation of the cells causes an elevation of the intracellular Ca2+ concentration, which in turn triggers granule fusion. Detailed discussion of ZG exocytosis can be found in the “Regulation of Physiologic and Pathologic Exocytosis in Pancreatic Acinar Cells” section by Dr. Herbert Y. Gaisano (21).
For ZG membrane proteins, major new findings from the recent studies came from the vesicular trafficking and small GTP-binding proteins groups (11, 12, 30, 63, 84). Among the small GTPase identified on ZG membrane, only Rab3D was previously reported on ZGs. In addition, punctate subapical staining of Rab11 just deep to the apical plasma membrane was observed in pancreatic acinar cells (29). The ZG localization of the majority of newly identified small G proteins including Rab27B, Rab11A, Rap1 and Rab6 were confirmed by immunocytochemistry at the isolated acini and ZG level (Figure 3). In subsequent functional studies, it was shown that Rab27B localizes on ZGs and plays an important role in regulating acinar exocytosis (10, 39). Furthermore, Rap1 was localized for the first time on pancreatic ZGs although it had previously been localized on parotid secretory granules and it was found that Rap1 activation plays a regulatory role in pancreatic amylase secretion (67). While Rab3D and Rab27B were present on all the ZGs, Rab6 and Rab11A localized to only a fraction of ZGs (Figure 3). This could indicate the existence of different subpopulations of ZGs. However, because Rabs can be extracted by corresponding GDIs from the membranes and cycles between membrane and cytosol, an alternative interpretation is that this represents ZGs at different stages in the secretory pathway. It is worth noting that Rab27A was later shown to be present in mouse acinar cell having a partial co-localization with ZGs and was required for digestive enzyme secretion (40). In addition to the small G proteins, we also found a novel SNARE protein, SNAP29, on ZGs. The ZG membrane localization of SNAP29 was confirmed by immunocytochemistry. Furthermore, it was found that SNAP29 and VAMP2 formed a complex on ZG membrane (82).
Another important category of molecules critical to vesicular trafficking and exocytosis is molecular motors and corresponding adaptors. It was reported previously that myosin Vc localized to the exocrine pancreas and largely overlaps with apical F-actin (64). Proteomics analysis identified the presence of myosin Vc on ZG membrane. It is of interest that Rab27a forms a complex with myosin Va on melanosome through a synaptotagmin-like linker protein, melanophilin. This complex is required to tether melanosomes to the actin cytoskeleton. In acinar cells, Rab27B, myosin Vc and at least two synaptotagmin-like proteins, slp1 and slp4, are present on ZG membrane. By analogy, Rab27B, slps and myosin Vc could form a complex on ZGs to regulate the tethering of ZGs at the apical membrane. In exocrine pancreas, targeting of ZGs to the apical cell surface requires an intact microtubule system and is then transferred to actin (27). The minus end-driven microtubular transport is thought mediated by molecular motor, dynein rather than kinesin. Purified ZGs were found to be associated with cytoplasmic dynein intermediate and heavy chain and to contain the major components of the dynein activator complex, dynactin. Consistent with this report, proteomics analysis identified a component of the dynactin complex, dynactin2/dynamitin from highly purified ZG membrane. Interestingly, it has been demonstrated that Rab6 functioned as a tethering factor controlling the recruitment of dynactin to membranes.
V. CONCLUSIONS AND FUTURE DIRECTIONS
The pancreatic ZG has been a prototypic model for all secretory granules in the regulated secretory cells. Since its discovery and initial morphological characterization by electron microscopy, information has been uncovered regarding its protein compositions and molecular mechanisms to govern its biogenesis, intracellular transport and regulated exocytosis. With the help from modern proteomics technologies, a very detailed molecular model of ZG content and membrane is being established. In the foreseeable future, a comprehensive molecular architecture of ZG will be developed including protein components, their membrane topologies, their copy numbers per ZG and protein complexes they are associated with. In addition, the lipid composition of the ZG membrane will be unveiled with the state-of-the-art lipidomic techniques. This comprehensive molecular view will facilitate a thorough mechanistic understanding on how the immature granules are formed from TGN and condensed into mature ZGs, as well as how they are released upon physiological stimulations.
VI. REFERENCES
- An SJ, Hansen NJ, Hodel A, Jahn R, and Edwardson JM. Analysis of the association of syncollin with the membrane of the pancreatic zymogen granule. J Biol Chem 275: 11306-11311, 2000. PMID: 10753942.
- Antonin W, Wagner M, Riedel D, Brose N, and Jahn R. Loss of the zymogen granule protein syncollin affects pancreatic protein synthesis and transport but not secretion. Mol Cell Biol 22: 1545-1554, 2002. PMID: 11839820.
- Aroso M, Agricola B, Hacker C, and Schrader M. Proteoglycans support proper granule formation in pancreatic acinar cells. Histochem Cell Biol 144: 331-346, 2015. PMID: 26105026.
- Arvan P, and Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J 332 ( Pt 3): 593-610, 1998. PMID: 9620860.
- Au CE, Bell AW, Gilchrist A, Hiding J, Nilsson T, and Bergeron JJ. Organellar proteomics to create the cell map. Curr Opin Cell Biol 19: 376-385, 2007. PMID: 17689063.
- Beaudoin AR, Grondin G, and Laperche Y. Immunocytochemical localization of gamma-glutamyltranspeptidase, GP-2 and amylase in the rat exocrine pancreas: the concept of zymogen granule membrane recycling after exocytosis. J Histochem Cytochem 41: 225-233, 1993. PMID: 7678269.
- Borta H, Aroso M, Rinn C, Gomez-Lazaro M, Vitorino R, Zeuschner D, Grabenbauer M, Amado F, and Schrader M. Analysis of low abundance membrane-associated proteins from rat pancreatic zymogen granules. J Proteome Res 9: 4927-4939, 2010. PMID: 20707389.
- Braun JE, and Scheller RH. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology 34: 1361-1369, 1995. PMID: 8606785.
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc 53: 211-354, 1978. PMID: 208670.
- Chen X, Li C, Izumi T, Ernst SA, Andrews PC, and Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun 323: 1157-1162, 2004. PMID: 15451418.
- Chen X, Ulintz PJ, Simon ES, Williams JA, and Andrews PC. Global topology analysis of pancreatic zymogen granule membrane proteins. Mol Cell Proteomics 7: 2323-2336, 2008. PMID: 18682380.
- Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, and Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics 5: 306-312, 2006. PMID: 16278343.
- Colomer V, Kicska GA, and Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem 271: 48-55, 1996. PMID: 8550606.
- Dartsch H, Kleene R, and Kern HF. In vitro condensation-sorting of enzyme proteins isolated from rat pancreatic acinar cells. Eur J Cell Biol 75: 211-222, 1998. PMID: 9587052.
- Doyle CJ, Yancey K, Pitt HA, Wang M, Bemis K, Yip-Schneider MT, Sherman ST, Lillemoe KD, Goggins MD, and Schmidt CM. The proteome of normal pancreatic juice. Pancreas 41: 186-194, 2012. PMID: 22129531.
- Edwardson JM, An S, and Jahn R. The secretory granule protein syncollin binds to syntaxin in a Ca2+-sensitive manner. Cell 90: 325-333, 1997. PMID: 9244306.
- Faust F, Gomez-Lazaro M, Borta H, Agricola B, and Schrader M. Rab8 is involved in zymogen granule formation in pancreatic acinar AR42J cells. Traffic 9: 964-979, 2008. PMID: 18363906.
- Freedman SD, and Scheele GA. Regulated secretory proteins in the exocrine pancreas aggregate under conditions that mimic the trans-Golgi network. Biochem Biophys Res Commun 197: 992-999, 1993. PMID: 7505580.
- Fukuda M, Kuroda TS, and Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 277: 12432-12436, 2002. PMID: 11856727.
- Fukuoka S, Freedman SD, and Scheele GA. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci U S A 88: 2898-2902, 1991. PMID: 2011597.
- Gaisano HY, Dolai S, and Takahashi T. Physiologic Exocytosis in Pancreatic Acinar Cells and Pathologic Fusion Underlying Pancreatitis. The Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc/2020.04.
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, and Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell 7: 2019-2027, 1996. PMID: 8970162.
- Gaisano HY, and Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology 136: 2040-2044, 2009. PMID: 19379751.
- Gaisano HY, Sheu L, Foskett JK, and Trimble WS. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J Biol Chem 269: 17062-17066, 1994. PMID: 7516331.
- Gaisano HY, Sheu L, Grondin G, Ghai M, Bouquillon A, Lowe A, Beaudoin A, and Trimble WS. The vesicle-associated membrane protein family of proteins in rat pancreatic and parotid acinar cells. Gastroenterology 111: 1661-1669, 1996. PMID: 8942747.
- Gerber SA, Rush J, Stemman O, Kirschner MW, and Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A 100: 6940-6945, 2003. PMID: 12771378.
- Geron E, Schejter ED, and Shilo BZ. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc Natl Acad Sci U S A 110: 10652-10657, 2013. PMID: 23754409.
- Goke B, Williams JA, Wishart MJ, and De Lisle RC. Low molecular mass GTP-binding proteins in subcellular fractions of the pancreas: regulated phosphoryl G proteins. Am J Physiol Cell Physiol 262: C493-500, 1992. PMID: 1539635.
- Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, and Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol Gastrointest Liver Phsyiol 270: G515-525, 1996. PMID: 8638719.
- Gomez-Lazaro M, Rinn C, Aroso M, Amado F, and Schrader M. Proteomic analysis of zymogen granules. Expert Rev Proteomics 7: 735-747, 2010. PMID: 20973645.
- Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, and Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol 27 Suppl 2: 27-32, 2012. PMID: 22320913.
- Guo Y, Sirkis DW, and Schekman R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol 30: 169-206, 2014. PMID: 25150009.
- Hammer JA, 3rd, and Wu XS. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr Opin Cell Biol 14: 69-75, 2002. PMID: 11792547.
- Hansen NJ, Antonin W, and Edwardson JM. Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell. J Biol Chem 274: 22871-22876, 1999. PMID: 10428873.
- Hofken T, Linder D, Kleene R, Goke B, and Wagner AC. Membrane dipeptidase and glutathione are major components of pig pancreatic zymogen granules. Exp Cell Res 244: 481-490, 1998. PMID: 9806799.
- Hooper NM, Cook S, Laine J, and Lebel D. Identification of membrane dipeptidase as a major glycosyl-phosphatidylinositol-anchored protein of the pancreatic zymogen granule membrane, and evidence for its release by phospholipase A. Biochem J 324: 151-157, 1997. PMID: 9164851.
- Hoops TC, and Rindler MJ. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem 266: 4257-4263, 1991. PMID: 1999417.
- Hou Y, Ernst SA, Lentz SI, and Williams JA. Genetic deletion of Rab27B in pancreatic acinar cells affects granules size and has inhibitory effects on amylase secretion. Biochem Biophys Res Commun 471: 610-615, 2016. PMID: 26845357.
- Hou Y, Ernst SA, Stuenkel EL, Lentz SI, and Williams JA. Rab27A Is Present in Mouse Pancreatic Acinar Cells and Is Required for Digestive Enzyme Secretion. PLoS One 10: e0125596, 2015. PMID: 25951179.
- Imamura T, Asada M, Vogt SK, Rudnick DA, Lowe ME, and Muglia LJ. Protection from pancreatitis by the zymogen granule membrane protein integral membrane-associated protein-1. J Biol Chem 277: 50725-50733, 2002. PMID: 12401800.
- Jacob M, Laine J, and LeBel D. Specific interactions of pancreatic amylase at acidic pH. Amylase and the major protein of the zymogen granule membrane (GP-2) bind to immobilized or polymerized amylase. Biochem Cell Biol 70: 1105-1114, 1992. PMID: 1284286.
- Lee J-S, Caruso JA, Hubbs G, Schnepp P, Woods J, Fang J, Li C, Zhang K, Stemmer PM, Jena B, and Chen X. Molecular architecture of mouse and human pancreatic zymogen granules: protein components and their copy numbers. Biophys Rep 4: 94-103, 2018. PMID: 29756009.
- Kalus I, Hodel A, Koch A, Kleene R, Edwardson JM, and Schrader M. Interaction of syncollin with GP-2, the major membrane protein of pancreatic zymogen granules, and association with lipid microdomains. Biochem J 362: 433-442, 2002. PMID: 11853552.
- Kelly ML, Abu-Hamdah R, Jeremic A, Cho SJ, Ilie AE, and Jena BP. Patch clamped single pancreatic zymogen granules: direct measurements of ion channel activities at the granule membrane. Pancreatology 5: 443-449, 2005. PMID: 15985770.
- Kirkpatrick DS, Gerber SA, and Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods 35: 265-273, 2005. PMID: 15722223.
- Kleene R, Dartsch H, and Kern HF. The secretory lectin ZG16p mediates sorting of enzyme proteins to the zymogen granule membrane in pancreatic acinar cells. Eur J Cell Biol 78: 79-90, 1999. PMID: 10099930.
- Kraemer J, Schmitz F, and Drenckhahn D. Cytoplasmic dynein and dynactin as likely candidates for microtubule-dependent apical targeting of pancreatic zymogen granules. Eur J Cell Biol 78: 265-277, 1999. PMID: 10350215.
- Kumazawa-Inoue K, Mimura T, Hosokawa-Tamiya S, Nakano Y, Dohmae N, Kinoshita-Toyoda A, Toyoda H, and Kojima-Aikawa K. ZG16p, an animal homolog of beta-prism fold plant lectins, interacts with heparan sulfate proteoglycans in pancreatic zymogen granules. Glycobiology 22: 258-266, 2012. PMID: 21948871.
- LeBel D, and Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun 154: 818-823, 1988. PMID: 2456764.
- Leblond FA, Viau G, Laine J, and Lebel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: implication of GP-2. Biochem J 291 ( Pt 1): 289-296, 1993. PMID: 8471046.
- MacDonald RJ, and Ronzio RA. Comparative analysis of zymogen granule membrane polypeptides. Biochem Biophys Res Commun 49: 377-382, 1972. PMID: 4640364.
- Messenger SW, Falkowski MA, and Groblewski GE. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium 55: 369-375, 2014. PMID: 24742357.
- Min BH, Jeong SY, Kang SW, Crabo BG, Foster DN, Chun BG, Bendayan M, and Park IS. Transient expression of clusterin (sulfated glycoprotein-2) during development of rat pancreas. J Endocrinol 158: 43-52, 1998. PMID: 9713325.
- Ohnishi H, Ernst SA, Wys N, McNiven M, and Williams JA. Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am J Physiol Gastrointest Liver Physiol 271: G531-538., 1996. PMID: 8843780.
- Ohnishi H, Samuelson LC, Yule DI, Ernst SA, and Williams JA. Overexpression of Rab3D enhances regulated amylase secretion from pancreatic acini of transgenic mice. J Clin Invest 100: 3044-3052, 1997. PMID: 9399951.
- Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell SK, Quinn DL, and Moore HP. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell 51: 1039-1051, 1987. PMID: 2826013.
- Owyang C and Williams J. Pancreatic Secretion. Gastroenterology, 4th Ed. Podolsky DS, Camillieri M, Fitz JG, Kalloo AN, Shanahan F, and Wang TC, Editors. Chapter 25. pp 340-366, 2015. DOI: 10.1002/9781118512074.ch25.
- Palade G. Intracellular aspects of the process of protein synthesis. Science 189: 347-358., 1975. PMID: 1096303.
- Petersen OH. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium 33: 337-344, 2003. PMID: 12765680.
- Petersen OH, and Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273-299, 2008. PMID: 17850212.
- Rindler MJ, and Hoops TC. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol 53: 154-163, 1990. PMID: 2076702.
- Rindler MJ, Xu CF, Gumper I, Smith NN, and Neubert TA. Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J Proteome Res 6: 2978-2992, 2007. PMID: 17583932.
- Rodriguez OC, and Cheney RE. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci 115: 991-1004, 2002. PMID: 11870218.
- Ronzio RA, Kronquist KE, Lewis DS, MacDonald RJ, Mohrlok SH, and O'Donnell JJ, Jr. Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim Biophys Acta 508: 65-84, 1978. PMID: 629968.
- Roussa E, Alper SL, and Thevenod F. Immunolocalization of anion exchanger AE2, Na+/H+ xchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J Histochem Cytochem 49: 463-474, 2001. PMID: 11259449.
- Sabbatini ME, Chen X, Ernst SA, and Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem 283: 23884-23894, 2008. PMID: 18577515.
- Sah RP, and Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol 27: 444-451, 2011. PMID: 21844752.
- Scheele GA. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem 250: 5375-5385, 1975. PMID: 1141235.
- Scheele GA, Fukuoka S, and Freedman SD. Role of the GP2/THP family of GPI-anchored proteins in membrane trafficking during regulated exocrine secretion. Pancreas 9: 139-149, 1994. PMID: 8190715.
- Scheele GA, Palade GE, and Tartakoff AM. Cell fractionation studies on the guinea pig pancreas. Redistribution of exocrine proteins during tissue homogenization. J Cell Biol 78: 110-130, 1978. PMID: 670290.
- Schrader M. Membrane targeting in secretion. Subcell Biochem 37: 391-421, 2004. PMID: 15376628.
- Strom M, Hume AN, Tarafder AK, Barkagianni E, and Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem 277: 25423-25430, 2002. PMID: 11980908.
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, and Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831-846, 2006. PMID: 17110340.
- Thévenod F. Channels and Transporters in Zymogen Granule Membranes and their Role in Granule Function: A Critical Assessment. The Pancreapedia: Exocrine Pancreas Knowledge Base. 2020. DOI: 10.3998/panc.2020.09.
- Valentijn JA, Gumkowski FD, and Jamieson JD. The expression pattern of rab3D in the developing rat exocrine pancreas coincides with the acquisition of regulated exocytosis. Eur J Cell Biol 71: 129-136, 1996. PMID: 8905289.
- Valentijn JA, Sengupta D, Gumkowski FD, Tang LH, Konieczko EM, and Jamieson JD. Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur J Cell Biol 70: 33-41., 1996. PMID: 8738417.
- Valentijn JA, Valentijn K, Pastore LM, and Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A 97: 1091-1095, 2000. PMID: 10655489.
- Wagner AC, Wishart MJ, Mulders SM, Blevins PM, Andrews PC, Lowe AW, and Williams JA. GP-3, a newly characterized glycoprotein on the inner surface of the zymogen granule membrane, undergoes regulated secretion. J Biol Chem 269: 9099-9104, 1994. PMID: 8132647.
- Walther TC, and Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol 190: 491-500, 2010. PMID: 20733050.
- Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, and Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell 7: 359-371, 2004. PMID: 15363411.
- Weng N, Thomas DD, and Groblewski GE. Pancreatic Acinar Cells Express Vesicle-associated Membrane Protein 2- and 8-Specific Populations of Zymogen Granules with Distinct and Overlapping Roles in Secretion. J Biol Chem 282: 9635-9645, 2007. PMID: 17272274.
- Williams JA. Receptor-mediated signal transduction pathways and the regulation of pancreatic acinar cell function. Curr Opin Gastroenterol 24: 573-579, 2008. PMID: 19122497.
- Williams JA, Chen X, and Sabbatini ME. Small G proteins as key regulators of pancreatic digestive enzyme secretion. Am J Physiol Endocrinol Metab 296: E405-414, 2009. PMID: 19088252.
- Williams JA, Groblewski GE, Ohnishi H, and Yule DI. Stimulus-secretion coupling of pancreatic digestive enzyme secretion. Digestion 1: 42-45, 1997. PMID: 9225090.
- Williams JA, and Yule DI. Stimulus-secretion Coupling in Pancreatic Acinar Cells. Physiology of the Gastrointestinal Tract. 5th Ed. Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD, Editors. Academic Press. Chapter 50. pp 1361-1398. 2012. ISBN: 9780123820266.
- Wishart MJ, Andrews PC, Nichols R, Blevins GT, Jr., Logsdon CD, and Williams JA. Identification and cloning of GP-3 from rat pancreatic acinar zymogen granules as a glycosylated membrane-associated lipase. J Biol Chem 268: 10303-10311, 1993. PMID: 8486693.
- Wu X, Wang F, Rao K, Sellers JR, and Hammer JA, 3rd. Rab27a is an essential component of melanosome receptor for myosin Va. Mol Biol Cell 13: 1735-1749, 2002. PMID: 12006666.
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, and Hammer JA, 3rd. Identification of an organelle receptor for myosin-Va. Nat Cell Biol 4: 271-278, 2002. PMID: 11887186.
- Yan W, Aebersold R, and Raines EW. Evolution of organelle-associated protein profiling. J Proteomics 72: 4-11, 2009. PMID: 19110081.
- Yu S, Michie SA, and Lowe AW. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. J Biol Chem 279: 50274-50279, 2004. PMID: 15385539.