Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2020.10
I. Introduction
While skin, liver and gut have the ability to regenerate and heal, other organs such as heart and brain do not display similar regenerative capacities. The adult pancreas displays a limited capacity to regenerate, although this regenerative capacity declines with age (17, 76-78, 86). Thus, with respect to the pancreas, the disagreement is not so much about the overall ability of the adult pancreas to regenerate, but rather which cells may act as cell(s) of origin in this process. For example, it is widely accepted that under physiologic conditions β-cell regeneration in the adult mouse pancreas originates from β-cell self-duplication (22, 79). However, depending on the type of injury model, it appears that new β-cells can arise from cells either residing within the ducts (1, 4, 18, 37, 94), in proximity to the ductal network (91), or from other pancreatic endocrine cells (15, 16, 81, 93). This uncertainty regarding the types of cells that may potentially give rise to new β-cells comes in part from the fact that in each experimental model of regeneration, the exact target cells and the severity of the injury are different. Here, we will first review some of the injury models that have been used to study the mechanisms leading to replacement of acinar- or β-cells, followed by a discussion of the discrepancies in these reports.
II. Injury Models to Study Pancreatic Regeneration
Over the years multiple models of pancreatic injury have been used by different investigators to explore the regenerative capacity of exocrine or endocrine compartments of the adult pancreas (48). Among these models, some of the commonly used are pancreatic duct ligation, partial pancreatectomy, caerulein-induced pancreatitis, alloxan- or streptozotocin-induced diabetes, and diphtheria toxin-mediated cell ablation. These models are of varying specificity, and they entail surgical, chemical or genetic methods.
A. Pancreatectomy (Px)
Pancreatectomy is the oldest model with which to examine the regenerative capacity of the pancreas (88). The first documented removal of the pancreas was performed on dogs by Johann C. Brunner in 1683 (32). However, it was first in 1890s that pancreatectomy was reported to result in diabetes, and hence a link between the pancreas and glucose homeostasis was established (32, 88). Px can be used to study acinar and β-cell recovery in both rats and mice, however because of the increased islet mass this injury model has been extensively used to study β-cell regeneration (5, 10, 20, 34, 49, 77, 85-87). Partial pancreatectomy (PPx) involves resection of less than 90% (often 50%-75%) of the adult mouse or rat pancreas (20-22, 44, 45, 63, 64, 86, 89). Here, the remnant of the pancreas displays normal gross morphology. A more severe form of Px that has been performed on rats is subtotal pancreatectomy (SPx), and entails removal of 90%-95% of the gland through tissue abrasion (3, 7, 49, 63, 71). Thus, in contrast to PPx, here the acinar cells in the remnant of the pancreas undergo rapid atrophy, which results in a desmoplastic reaction (49, 53). Regardless of the extent of resection, pancreatectomy is associated with an IGF/PI3K-dependent up-regulation of Pdx1 expression in duct cells (71, 85, 86). Interestingly, the limited organ recovery that follows Px appears to be proportional to the size of the excision (3, 7, 46, 63, 65). Subtotal pancreatectomy in rats leads to ductal cell proliferation and induction of an extensive regenerative process that promotes mature duct cells to regress and re-express embryonic genes such as Pdx1, Ptf1a, and Ngn3 before differentiating to the different pancreatic cell types (49, 71). While these data imply that the duct cells might contribute to the observed increased islet mass following Px, other studies would argue against the involvement of duct cells in this process (21, 22, 45, 89). Using lineage tracing studies, independent investigators have not been able to find any evidence for β-cell neogenesis following 50%-75% PPx (22, 89). Accordingly, 50% PPx in Ngn3-GFP transgenic mice failed to induce Ngn3-expression in cells within islets or ducts (45). All together, although the potential contribution of other cell types cannot be ruled out, the current literature supports the notion that the main source for acinar or β-cell regeneration that follows Px is pre-existing acinar or β-cells, respectively (21, 22, 57, 89).
B. Pancreatic duct ligation (PDL)
As in the case of Px, ductal obstruction and ligation have historically been used in investigating pancreatic regeneration (88). PDL involves ligation of one of the main ducts, which leads to acinar cell death and inflammation in the area distal to the ligation. An advantage with PDL is that the unligated portion of the pancreas remains unaffected, and thus can be used as an internal control. However, the regenerative process in this model, particularly the acinar regeneration, appears to be species-dependent. PDL in rats is associated with near complete acinar recovery through a process that involves appearance of ductular structures, and their differentiation into acinar cells (7, 13). In mice, although PDL results in the formation of similar metaplastic ducts, the acinar compartment does not regenerate (13, 21, 40, 73). Lineage tracing studies in mice show that both surviving acinar cells (21) and Hnf1β-expressing duct cells (73) in the ligated part of the pancreas can contribute to the formation of these ductular structures. In other words, the tubular structures observed in the ligated part consist of acinar-derived metaplastic ducts and pre-existing ducts that have changed morphology. Similarly, in rats it is believed that pre-existing acinar cells transdifferentiate into these ductal structures (7, 13), whereas the contribution of duct cells in this process has yet to be determined.
Pancreatic duct ligation has been primarily used to provide insights on islet β-cell generation, as it is reported to stimulate β-cell regeneration in both mouse and rat (7, 13, 83, 84, 89-91, 94). Nevertheless, there is a controversy with respect to the mechanism allowing the observed β-cell generation. While some studies favor β-cell proliferation as the main mechanism for β-cell formation after PDL (13, 40, 67, 73, 89), others support the potential contribution of non β-cells (in particular cells within or in proximity of ductal network) to β-cell neogenesis in a PDL setting (37, 62, 91, 94).
C. Caerulein-induced pancreatitis
Caerulein is a cholecystokinin orthologue, which when administered repeatedly at high concentrations leads to the death and dropout of over half of the acinar compartment which can cause acute or chronic pancreatitis (26, 42, 48). In mice, the pancreas regains its normal histology within a week after caerulein treatment. The rapid regenerative process associated with this injury model has been used by many investigators to follow the course of acinar recovery (29, 35, 38, 55, 74). Lineage-tracing studies have shown that following caerulein-induced pancreatitis the surviving acinar cells contribute to the recovery of the acinar compartment (21, 29, 55, 74). Acinar regeneration in this model is through acinar-to-ductal metaplasia (ADM), a process that requires a transient reactivation of various developmental genes and signaling pathways, including notch, hedgehog and wnt (29, 38, 41, 55, 56, 66). ADM involves dedifferentiation of acinar cells into duct-like cells, proliferation of metaplastic ducts, and finally re-differentiation of duct-like cells into acinar cells (55). A first step toward transdifferentiation of one cell type to another cell type is that cells have to lose their original identity in order to acquire a new one. Accordingly, the dedifferentiation of acinar cells is associated with expression of ductal markers such as Hnf6, Sox9, and cytokeratin-19 and concomitant repression of acinar markers Ptf1a, Mist1, amylase and Cpa (66). Wnt/β-catenin signaling is one of the embryonic pathways, which is reactivated in ADM following caerulein-induced pancreatitis (55, 56). However, for the ADM to re-differentiate into acinar cells wnt signaling has to be eventually downregulated, as persistent wnt/β-catenin activity leads to impaired acinar recovery (25, 55). The precise mechanism for the dynamic activity of wnt/β-catenin in ADM is not clear, but a recent report implicates HDACs as an important epigenetic switch required for controlling nuclear β-catenin transcriptional activity (25). The transcriptional factor PDX1 has primarily been associated with the embryonic pancreas and mature β-cells in the adult pancreas. However, a more recent study highlights the importance of PDX1 in maintaining acinar cell identity (68). PDX1 displays similar dynamic expression as wnt/β-catenin during ADM, and accordingly its down-regulation is necessary for re-differentiation of ADM into acinar cells (68).
D. Other models of pancreatitis
In addition to the aforementioned caerulein-induced pancreatitis, other rodent models commonly used to study acute pancreatitis entail bile salt infusion, duct obstruction, the choline-deficient ethionine supplemented diet (CDE), or administration of basic amino acids such as L-arginine. These injury models have been extensively described and reviewed by Lerch and Gorelick elsewhere (48).
E. Alloxan- or streptozotocin-induced diabetes
Alloxan and streptozotocin (STZ) are used to induce diabetes by chemical ablation of pancreatic β-cells. Alloxan was first described in the early 1800s, but its diabetogenic property was reported in 1943, and since then alloxan treatment has been used as an experimental model for diabetes (75). STZ was initially used as a chemotherapeutic agent in pancreatic islet cell tumors and other malignancies (47), but since its discovery as a diabetogenic agent in 1963, it has been widely used in diabetes research (27). Alloxan and STZ are both toxic glucose analogues that preferentially accumulate in insulin producing β-cells via the Glut2 glucose transporter (47). Diabetes as the result of alloxan or STZ-treatment is not associated with β-cell regeneration (75, 89). Because of the absence of spontaneous β-cell recovery, these models have been useful tools to study a given treatment on β-cell regeneration. In addition, alloxan- or STZ-treatment can be combined with pancreatic duct ligation to study the effect of hyperglycemia on the regenerative process in the ligated portion of the pancreas (13, 16, 62). While the combination of alloxan- or STZ-treatment and PDL in mice led to transformation of glucagon-producing α-cells or acinar cells into β-cells (16, 62), no such α-to β-cell conversion could be found when rats were subjected to a combined PDL and STZ-treatment (13).
F. Diphtheria toxin-mediated cell ablation
A relatively new method which enables cell-specific ablation is transgenic activation of the diphtheria toxin cell death pathway using a cell-specific promoter (8, 61). Mature diphtheria toxin (DT) is composed of subunits A and B (DTA and DTB) (33, 82). DT binds a toxin receptor on the cell surface of toxin-sensitive cells and is endocytosed (24, 31, 69). Upon entry into the cytoplasm, the DTA subunit is released and it catalyzes the inactivation of elongation factor 2, resulting in termination of all protein synthesis, with rapid apoptotic death of the target cell (11, 36). The toxicity of DTA is sufficiently high that only one molecule of DTA in the cytosol may be enough to kill the cell (92). The DT receptor (DTR) is a membrane-anchored form of the heparin binding EGF-like growth factor (HB-EGF precursor) (58). The human and simian HB-EGF precursors bind DT and function as toxin receptors, whereas HB-EGF from mice and rats do not bind the toxin and therefore remain insensitive to DT (54). Thus, transgenic expression of the simian or human DTR in mice can render naturally DT-resistant mouse cells DT-sensitive (14, 39, 69). Recently, a mouse strain was generated (R26DTR), in which a loxP-flanked STOP cassette and the open reading frame of simian DTR had been introduced into the ROSA26 locus (11). In the R26DTR strain, the gene encoding DTR is under the control of the potent Rosa promoter, but DTR expression is dependent first on Cre-recombinase removal of the STOP cassette (11). Following Cre-recombinase activity, the DTR-expressing cells, i.e. cells expressing Cre, and all of their progeny, are viable and function normally. However, these cells are rapidly killed upon DT administration. Noteworthy, the HB-EGF is no longer active as an EGFR ligand, as transgenic lines expressing DTR in different pancreatic lineages do not display any abnormal phenotype (17, 18). In the adult pancreas, DTR/DTA-mediated β-cell ablation has been used to study regeneration following α- or β-cell specific losses (15, 17, 59, 72, 81), acinar (17, 18), or acinar and endocrine cell ablation (17, 18).
III. The Inconsistencies
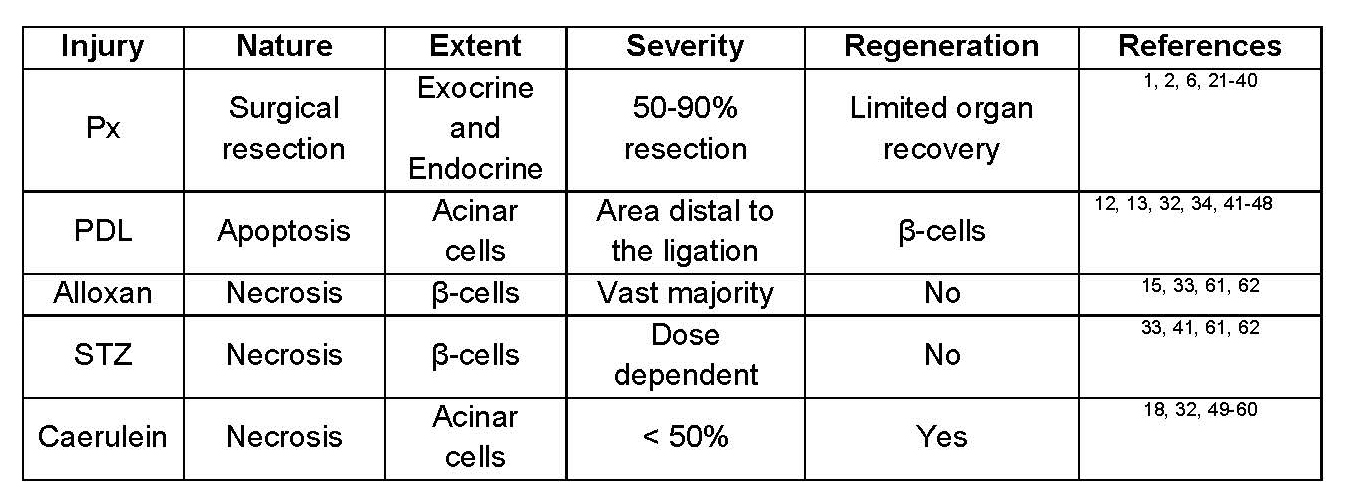
A brief look at table 1 highlights the inconsistencies that currently exist in the literature regarding the regenerative capacity of the adult mouse pancreas. For example, there is a complete recovery of the acinar compartment within a week after caerulein-induced pancreatitis, whereas there is principally no acinar regeneration following PDL. Additionally, α-cells can differentiate into β-cells, however this plasticity has been observed only upon total β-cell ablation but not following partial loss of β-cell mass. Variations between different studies are likely due to the disparities in the nature, extent, and perhaps more importantly the severity of injury used. In this review, we will argue that the combined effects of these parameters not only may determine whether or not regeneration would occur, but also dictate which cell type(s) should contribute to this process.
Table 1. Response of mouse pancreas to different damage regimens.
A. The nature of injury
Here, the question is not so much about whether the injury is chemically, mechanically or genetically induced, but rather what kind of cell death does it trigger? Apoptosis is programmed cell death generally associated with retention of plasma membrane integrity, condensation and cleavage of nuclear and cytoplasmic proteins and cell shrinkage or the formation of apoptotic bodies (28). Apoptosis is a highly coordinated process which requires significant amount of energy, and therefore relies on mitochondrial respiration and ATP production (23). Necrosis, on the other hand is invoked in response to external stimuli and ATP-deficiency (23). Pancreatic injury and ensuing regeneration invariably depend on proper clearance of the dead cells (17). Because of its nature (loss of cytoplasmic membrane integrity, cellular fragmentation and release of lysosomal and granular contents into surrounding extracellular space), necrosis does not allow for proper removal of cell organelles, and as the result it is followed by reactive inflammation (2, 12, 23, 52, 60, 70). In contrast, apoptosis involves debriding the tissue without generating massive inflammation that is usually induced by the degeneration of dead cells (23). The effect of apoptosis or necrosis on regeneration is perhaps best manifested when one compares β-cell regeneration following STZ (or alloxan) treatment with DT-mediated β-cell ablation. In these two models, the target cells (β-cells) as well as the degree of β-cell loss (75-80% sub-optimal condition for STZ, or 75% β-cell ablation using PdxCreERT) are similar (17, 22). Interestingly, STZ-induced necrosis is accompanied with a massive inflammatory response and the absence of β-cell regeneration, whereas DT-induced apoptosis leads to almost complete β-cell mass recovery (59).
Overall, compared to the necrosis, apoptosis provides an environment that would favor β-cell regeneration. Notably, acinar regeneration appears to be less sensitive to the nature of injury, as robust acinar tissue recovery have been reported in both necrotic (caerulein) as well as apoptotic (DT-mediated) environments (17, 18, 29, 35, 38, 55, 56, 74).
B. The extent of injury
Another factor that may influence the regenerative process is the extent of injury. In other words, how many different cell types are affected by the insult? In addition to the ductal, acinar and five different hormone-producing endocrine cell types, the adult pancreas is home to numerous endothelial, stellate and neuronal cells. The type of injured cell types is important as recent reports have shed light on the role of macrophages in inducing acinar-to-ductal metaplasia, as well as promoting β-cell, or acinar cell regeneration (9, 17, 19, 51, 90). Macrophages appear to have differential functions in diverse phases of regeneration, first to debride the tissue following injury, and secondly to convert injury signals into lineage-specific regenerative signals (6, 17, 50). One exception to this rule is PDL, which as mentioned earlier is associated with acinar atrophy. Thus, one would expect that this model would lead to acinar regeneration, but instead it stimulates β-cell regeneration. Clearly, the effect of PDL on non-acinar cells residing in the ligated part cannot be ruled out. Therefore, it is possible that the combined regenerative signals released by macrophages (as the result of engulfing damaged acinar- and non-acinar cells) would serendipitously create an environment that would promote β-cell regeneration instead of acinar. In fact, combined PDL and β-cell ablation by STZ has been reported to enhance acinar to β-cell transdifferentiation (62).
Based on our current understanding of the involvement of macrophages in regeneration, simultaneous ablation of many cell types would make direct interpretation of cellular mechanism of regeneration difficult. Thus, cell-type specific ablation may be a better method for analyzing the in vivo function of cells during regeneration, which can be achieved by using streptozotocin, alloxan (for β-cells) or caerulein (for acinar cells). Alternatively, transgenic activation of the diphtheria toxin (DTR/DTA) cell death pathway, which depending on the promoter, can target one specific (for example Elastase promoter for acinar cell ablation) or more than one cell type (Pdx1 promoter to target all pancreatic epithelial cells) (17, 18).
C. The severity of injury
Mounting evidence suggests that the severity of injury is perhaps one of the most important elements that dictate whether the mechanism for repair should include replication of pre-existing cells, or neogenesis from other cell types. DT-mediated cell ablation has been used by number of investigators to vary the extent and the severity of injury, while keeping other variables (such as the nature of the injury) relatively constant. Collectively, it appears that regardless of cell type, as long as ablation of a specific cell type does not reach near 100%, the mechanism for regeneration mainly involves the pre-existing cells (17, 22, 29, 55, 74, 81). Therefore, following 75% ablation of β-cells, surviving β-cells proliferate to generate new β-cells, whereas complete loss of insulin-producing cells promotes conversion of other endocrine cell types into β-cells (15, 17, 22, 30, 80, 81). Consistently, acinar cell recovery following caerulein-induced pancreatitis is through pre-existing acinar cells. However, near complete loss of both acinar as well as endocrine cells stimulates cells within the ductal compartment to form new acinar and endocrine cells (17, 18). One could also argue that the extent of the surgical intervention may be important also in the pancreatectomy setting, and could explain discrepancies in some reports describing absence or vigorous pancreatic regeneration after partial or subtotal pancreatectomy, respectively (3, 22, 49). However, as mentioned earlier unlike PPx, subtotal pancreatectomy is associated with acinar atrophy and a desmoplastic reaction. Of note, this inflammatory reaction has been reported to be important for the robust regeneration that follows SPx, as inhibition of the inflammation prevented regeneration (7, 43). Therefore, it is likely that the inconsistencies between PPx and SPx are due to the presence or the absence of inflammation rather than the extent of injury.
IV. Conclusions
Pancreatic regeneration relies on a complex interaction between cells that provide necessary regenerative signals and cells that are receptive to those signals. As discussed here, the nature, extent and the severity of injury are three important parameters that determine whether tissue recovery is achieved. β-cell regeneration seems to be more sensitive to the nature of injury than acinar regeneration. Finally, the extent of injury determines which cell types would respond to these regenerative signals.
V. Acknowledgements
Supported by NIH/NIDDK grants DK101413-01A1, and DK103002-01.
VI. References
- Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, Lacas-Gervais S, Ambrosetti D, Benizri E, Hecksher-Sorensen J, Gounon P, Ferrer J, Gradwohl G, Heimberg H, Mansouri A, and Collombat P. Adult Duct-Lining Cells Can Reprogram into beta-like Cells Able to Counter Repeated Cycles of Toxin-Induced Diabetes. Dev Cell 26: 86-100, 2013. PMID: 23810513.
- Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189-196, 2004. PMID: 14715516.
- Bonner-Weir S, Baxter LA, Schuppin GT, and Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42: 1715-1720, 1993. PMID: 8243817.
- Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, and Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans 36: 353-356, 2008. PMID: 18481956.
- Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, and Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 5 Suppl 2: 16-22, 2004. PMID: 15601370.
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, and Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572-579, 2012. PMID: 22388089.
- Bouwens L. Beta cell regeneration. Curr Diabetes Rev 2: 3-9, 2006. PMID: 18220612.
- Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, and Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science 238: 1563-1565, 1987. PMID: 3685993.
- Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, and Powers AC. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab 19: 498-511, 2014. PMID: 24561261.
- Brockenbrough JS, Weir GC, and Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37: 232-236, 1988. PMID: 3292318.
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, and Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419-426, 2005. PMID: 15908920.
- Buja LM, Eigenbrodt ML, and Eigenbrodt EH. Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med 117: 1208-1214, 1993. PMID: 8250690.
- Cavelti-Weder C, Shtessel M, Reuss JE, Jermendy A, Yamada T, Caballero F, Bonner-Weir S, and Weir GC. Pancreatic duct ligation after almost complete beta-cell loss: exocrine regeneration but no evidence of beta-cell regeneration. Endocrinology 154: 4493-4502, 2013. PMID: 24029238.
- Cha JH, Chang MY, Richardson JA, and Eidels L. Transgenic mice expressing the diphtheria toxin receptor are sensitive to the toxin. Mol Microbiol 49: 235-240, 2003. PMID: 12823824.
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, and Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature 514: 503-507, 2014. PMID: 25141178.
- Chung CH, Hao E, Piran R, Keinan E, and Levine F. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells 28: 1630-1638, 2010. PMID: 20653050.
- Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, and Esni F. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and beta-cell regeneration in mice. Gastroenterology 147: 1106-1118 e1111, 2014. PMID: 25128759.
- Criscimanna A, Speicher JA, Houshmand G, Shiota C, Prasadan K, Ji B, Logsdon CD, Gittes GK, and Esni F. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology 141: 1451-1462, 1462 e1451-1456, 2011. PMID: 21763240.
- Cruz AF, Rohban R, and Esni F. Macrophages in the pancreas: Villains by circumstances, not necessarily by actions. Immun Inflamm Dis 8: 807-824, 2020. PMID: 32885589.
- De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, and Stoffers DA. Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52: 365-371, 2003. PMID: 12540609.
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, and Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117: 971-977, 2007. PMID: 17404620.
- Dor Y, Brown J, Martinez OI, and Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41-46, 2004. PMID: 15129273.
- Dorn GW, 2nd. Molecular mechanisms that differentiate apoptosis from programmed necrosis. Toxicol Pathol 41: 227-234, 2013. PMID: 23222994.
- Drazin R, Kandel J, and Collier RJ. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem 246: 1504-1510, 1971. PMID: 5545093.
- Eisses JF, Criscimanna A, Dionise ZR, Orabi AI, Javed TA, Sarwar S, Jin S, Zhou L, Singh S, Poddar M, Davis AW, Tosun AB, Ozolek JA, Lowe ME, Monga SP, Rohde GK, Esni F, and Husain SZ. Valproic Acid Limits Pancreatic Recovery after Pancreatitis by Inhibiting Histone Deacetylases and Preventing Acinar Redifferentiation Programs. Am J Pathol 185: 3304-3315, 2015. PMID: 26476347.
- Eisses JF, Davis AW, Tosun AB, Dionise ZR, Chen C, Ozolek JA, Rohde GK, and Husain SZ. A computer-based automated algorithm for assessing acinar cell loss after experimental pancreatitis. PLoS One 9: e110220, 2014. PMID: 25343460.
- Eleazu CO, Eleazu KC, Chukwuma S, and Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord 12: 60, 2013. PMID: 24364898.
- Erwig LP, and Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol 171: 2-8, 2007. PMID: 17591947.
- Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, and Maitra A. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology 135: 621-631, 2008. PMID: 18515092.
- Furuyama K, Chera S, van Gurp L, Oropeza D, Ghila L, Damond N, Vethe H, Paulo JA, Joosten AM, Berney T, Bosco D, Dorrell C, Grompe M, Raeder H, Roep BO, Thorel F, and Herrera PL. Diabetes relief in mice by glucose-sensing insulin-secreting human alpha-cells. Nature 567: 43-48, 2019. PMID: 30760930.
- Gill DM, and Dinius LL. Observations on the structure of diphtheria toxin. J Biol Chem 246: 1485-1491, 1971. PMID: 5545090.
- Granger A, and Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med 266: 325-338, 2009. PMID: 19765178.
- Greenfield L, Bjorn MJ, Horn G, Fong D, Buck GA, Collier RJ, and Kaplan DA. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A 80: 6853-6857, 1983. PMID: 6316330.
- Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, and Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol 66: 163-174, 2003. PMID: 12846556.
- Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, and Konieczny SF. Extensive Pancreas Regeneration Following Acinar-Specific Disruption of Xbp1 in Mice. Gastroenterology 141: 1463-1472, 2011. PMID: 21704586.
- Honjo T, Nishizuka Y, Kato I, and Hayaishi O. Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J Biol Chem 246: 4251-4260, 1971. PMID: 3426212.
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, and Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 105: 19915-19919, 2008. PMID: 19052237.
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, and Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128: 728-741, 2005. PMID: 15765408.
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, and Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17: 211-220, 2002. PMID: 12196292.
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, and Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138: 653-665, 2011. PMID: 21266405.
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, and Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22: 737-750, 2012. PMID: 23201164.
- Lampel M, and Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 373: 97-117, 1977. PMID: 139754.
- Lampeter EF, Gurniak M, Brocker U, Klemens C, Tubes M, Friemann J, and Kolb H. Regeneration of beta-cells in response to islet inflammation. Exp Clin Endocrinol Diabetes 103 Suppl 2: 74-78, 1995. PMID: 8839258.
- Leahy JL, Bonner-Weir S, and Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest 81: 1407-1414, 1988. PMID: 3284912.
- Lee CS, De Leon DD, Kaestner KH, and Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 55: 269-272, 2006. PMID: 16361411.
- Lehv M, and Fitzgerald PJ. Pancreatic acinar cell regeneration. IV. Regeneration after resection. Am J Pathol 53: 513-535, 1968. PMID: 5677136.
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51: 216-226, 2008. PMID: 18087688.
- Lerch MM, and Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 144: 1180-1193, 2013. PMID: 23622127.
- Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, and Bonner-Weir S. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 123: 2792-2802, 2010. PMID: 20663919.
- Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, and Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194-4199, 2010. PMID: 20160075.
- Liou GY, Doppler H, Necela B, Krishna M, Crawford HC, Raimondo M, and Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol 202: 563-577, 2013. PMID: 23918941.
- Majno G, and Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146: 3-15, 1995. PMID: 7856735.
- Migliorini RH. Two-stage procedure for total pancreatectomy in the rat. Diabetes 19: 694-697, 1970. PMID: 5474205.
- Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, and Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 270: 1015-1019, 1995. PMID: 7836353.
- Morris JPt, Cano DA, Sekine S, Wang SC, and Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 120: 508-520, 2010. PMID: 20071774.
- Morris JPt, Wang SC, and Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 10: 683-695, 2010. PMID: 20814421.
- Murtaugh LC, and Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol 77: 229-249, 2015. PMID: 25386992.
- Naglich JG, Metherall JE, Russell DW, and Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69: 1051-1061, 1992. PMID: 1606612.
- Nir T, Melton DA, and Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553-2561, 2007. PMID: 17786244.
- Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608-627, 2003. PMID: 12620919.
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, and Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50: 435-443, 1987. PMID: 3649277.
- Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, and Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140: 751-764, 2013. PMID: 23325761.
- Pearson KW, Scott D, and Torrance B. Effects of partial surgical pancreatectomy in rats. I. Pancreatic regeneration. Gastroenterology 72: 469-473, 1977. PMID: 832795.
- Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, and Jetton TL. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 55: 3289-3298, 2006. PMID: 17130472.
- Plachot C, Movassat J, and Portha B. Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto-Kakizaki rat, a spontaneous model of type II diabetes. Histochem Cell Biol 116: 131-139, 2001. PMID: 11685541.
- Prevot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J, Bertrand L, Heimberg H, Konieczny SF, Dor Y, Lemaigre FP, and Jacquemin P. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut 61: 1723-1732, 2012. PMID: 22271799.
- Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, and Kushner JA. beta-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 62: 1634-1645, 2013. PMID: 23349489.
- Roy N, Takeuchi KK, Ruggeri JM, Bailey P, Chang D, Li J, Leonhardt L, Puri S, Hoffman MT, Gao S, Halbrook CJ, Song Y, Ljungman M, Malik S, Wright CV, Dawson DW, Biankin AV, Hebrok M, and Crawford HC. PDX1 dynamically regulates pancreatic ductal adenocarcinoma initiation and maintenance. Genes Dev 30: 2669-2683, 2016. PMID: 28087712.
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, and Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 19: 746-750, 2001. PMID: 11479567.
- Scaffidi P, Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191-195, 2002. PMID: 12110890.
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, and Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48: 507-513, 1999. PMID: 10078550.
- Shiota C, Prasadan K, Guo P, El-Gohary Y, Wiersch J, Xiao X, Esni F, and Gittes GK. Alpha-cells are dispensable in postnatal morphogenesis and maturation of mouse pancreatic islets. Am J Physiol Endocrinol Metab 305: E1030-1040, 2013. PMID: 23982158.
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, and Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 17: 849-860, 2009. PMID: 20059954.
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, and Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133: 1999-2009, 2007. PMID: 18054571.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50: 537-546, 2001. PMID: 11829314.
- Takahashi H, Okamura D, Starr ME, Saito H, and Evers BM. Age-dependent reduction of the PI3K regulatory subunit p85alpha suppresses pancreatic acinar cell proliferation. Aging Cell 11: 305-314, 2012. PMID: 22212451.
- Tanigawa K, Nakamura S, Kawaguchi M, Xu G, Kin S, and Tamura K. Effect of aging on B-cell function and replication in rat pancreas after 90% pancreatectomy. Pancreas 15: 53-59, 1997. PMID: 9211493.
- Teta M, Long SY, Wartschow LM, Rankin MM, and Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 54: 2557-2567, 2005. PMID: 16123343.
- Teta M, Rankin MM, Long SY, Stein GM, and Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817-826, 2007. PMID: 17488631.
- Thorel F, Damond N, Chera S, Wiederkehr A, Thorens B, Meda P, Wollheim CB, and Herrera PL. Normal Glucagon Signaling and {beta}-Cell Function After Near-Total {alpha}-Cell Ablation in Adult Mice. Diabetes 60: 2872-2882, 2011. PMID: 21926270.
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, and Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464: 1149-1154, 2010. PMID: 20364121.
- Tsuneoka M, Nakayama K, Hatsuzawa K, Komada M, Kitamura N, and Mekada E. Evidence for involvement of furin in cleavage and activation of diphtheria toxin. J Biol Chem 268: 26461-26465, 1993. PMID: 8253774.
- Van de Casteele M, Leuckx G, Cai Y, Yuchi Y, Coppens V, De Groef S, Van Gassen N, Baeyens L, Heremans Y, Wright CV, and Heimberg H. Partial duct ligation: beta-cell proliferation and beyond. Diabetes 63: 2567-2577, 2014. PMID: 25060885.
- Wang RN, Kloppel G, and Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38: 1405-1411, 1995. PMID: 8786013.
- Watanabe H, Saito H, Nishimura H, Ueda J, and Evers BM. Activation of phosphatidylinositol-3 kinase regulates pancreatic duodenal homeobox-1 in duct cells during pancreatic regeneration. Pancreas 36: 153-159, 2008. PMID: 18376306.
- Watanabe H, Saito H, Rychahou PG, Uchida T, and Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology 128: 1391-1404, 2005. PMID: 15887120.
- Watanabe H, Saito H, Ueda J, and Evers BM. Regulation of pancreatic duct cell differentiation by phosphatidylinositol-3 kinase. Biochem Biophys Res Commun 370: 33-37, 2008. PMID: 18339306.
- Weaver CV, and Garry DJ. Regenerative biology: a historical perspective and modern applications. Regen Med 3: 63-82, 2008. PMID: 18154463.
- Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, Paredes J, Welsh C, Wiersch J, and Gittes GK. No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest 123: 2207-2217, 2013. PMID: 23619362.
- Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C, and Gittes GK. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci U S A 111: E1211-1220, 2014. PMID: 24639504.
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, and Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197-207, 2008. PMID: 18243096.
- Yamaizumi M, Uchida T, Okada Y, and Furusawa M. Neutralization of diphtheria toxin in living cells by microinjection of antifragment A contained within resealed erythrocyte ghosts. Cell 13: 227-232, 1978. PMID: 627034.
- Yang YP, Thorel F, Boyer DF, Herrera PL, and Wright CV. Context-specific {alpha}-to-{beta}-cell reprogramming by forced Pdx1 expression. Genes Dev 25: 1680-1685, 2011. PMID: 21852533.
- Zhang M, Lin Q, Qi T, Wang T, Chen CC, Riggs AD, and Zeng D. Growth factors and medium hyperglycemia induce Sox9+ ductal cell differentiation into beta cells in mice with reversal of diabetes. Proc Natl Acad Sci U S A 113: 650-655, 2016. PMID: 26733677.