Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2014.10
1. Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas was first reported by Ohhashi et al. in 1982 as mucin producing pancreatic cancer which had better prognosis compared with conventional pancreatic ductal adenocarcinoma (PDAC) (14). IPMN’s action show a wide spectrum of histological grad from low, intermediate, and high grade dysplasia to invasive carcinoma it has also been also reported that the patients with IPMNs have a high prevalence of distinct neoplastic lesions including concomitant PDAC (Figure 1) and extrapancreatic malignancies such as colorectal, breast, and prostate cancer (9, 21, 23, 24, 27, 28, 30, 31). Because of its unique characteristics, numerous investigations have been carried out to date, and the international consensus guidelines were edited in 2006 (Sendai guidelines), then revised in 2012 (Fukuoka guidelines), for the adequate management of IPMN. In this article, characteristics, diagnosis, and management of IPMN are described based on the Fukuoka guidelines 2012 (26, 27).

Figure 1. Pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. Enhanced computed tomography shows irregular low density solid lesion in the pancreatic body (arrow head) and cystic lesion in the pancreatic tail (arrow), indicating ductal adenocarcinoma in the pancreas body concomitant with intraductal papillary mucinous neoplasm in the pancreatic tail.
2. Etiology and Symptoms
IPMNs are predominantly observed in the aged male patients. Most patients are asymptomatic, while some patients experience symptoms of abdominal pain / discomfort, back pain, pancreatitis, jaundice, new onset or deterioration of diabetes, and elevated serum carbohydrate antigen 19-9 (CA19-9) level (27). Pancreatitis and jaundice are reported to be predictors for the malignant IPMN, and new onset or deterioration of diabetes and elevated serum CA19-9 level are predictors for the possible presence of concomitant PDAC (2, 6, 11).
3. Morphological type
IPMNs are morphologically classified into 3 types, namely, main duct type (MD-IPMN), branch duct type (BD-IPMN), and mixed type involving both main duct and branch duct (27). Fukuoka guidelines defined MD-IPMN as the diffuse or localized lesion having dilation of main pancreatic duct (MPD) of greater than 5mm in diameter (Figure 2), without any significant lesion except for IPMN (27). The morphological type of IPMN is usually determined at the initial imaging assessment because morphological typing is one of the important issues for the adequate management strategy of IPMNs and most patients with IPMNs are surveyed without resection and therefore pathological assessment cannot be carried out (27). Magnetic resonance cholangiopancreatography (MRCP) is usually suitable for the assessment of the morphological type of IPMN (Figure 3) (27).
4. Imaging Assessment
Standard imaging modalities for the assessment for IPMNs include enhanced computed tomography (CT), magnetic resonance imaging (MRI) / MRCP, and endoscopic ultrasound (EUS) (2). CT has advantages in terms of familiarity for many physicians, fast examination time with objective spatial resolution, and has roles for the assessments of local invasion of IPMN, preoperative vascular anatomy, and extrapancreatic malignancies (7). MRCP is suitable for the assessment of whole pancreatic ductal system (thus, morphological type of IPMN is determined by MRCP as described above) (Figure 3) and for the recognition of the morphological changes of IPMN as well as MPD during surveillance (7). EUS has roles for the assessment of the presence of mural nodule in IPMN (Figure 4), and for the early diagnosis of concomitant PDAC during surveillance, however, EUS cannot always be performed with high quality in every institution (8, 27).

Figure 2. Dilated orifice of duodenal papilla. Dilated orifice of the duodenal papilla by mucin hyper secretion is one of the representative features of intraductal papillary mucinous neoplasm of the pancreas.
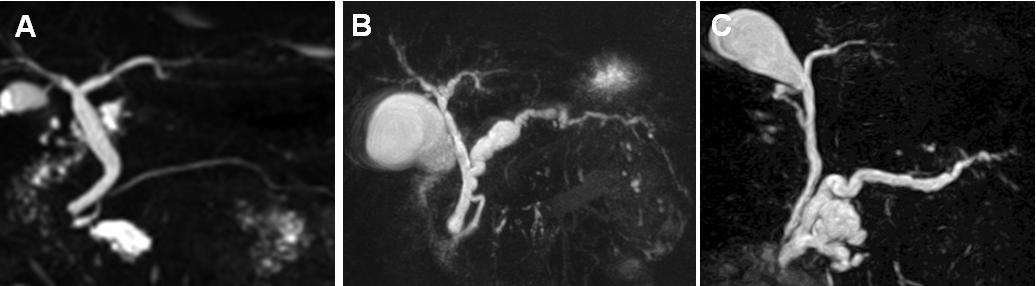
Figure 3. Morphological types of intraductal papillary mucinous neoplasm of the pancreas. Magnetic resonance cholangiopancreatography demonstrates branch duct type (A), main duct type (B), and mixed type (C) of intraductal papillary mucinous neoplasm of the pancreas.
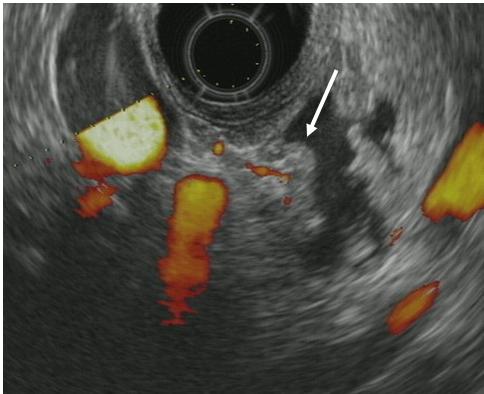
Figure 4. Mural nodule in the cyst. Endoscopic ultrasonography shows mural nodule (arrow) having blood flow in the cystic lesion.
5. Assessment of Pancreatic Juice / Cyst Fluid
Routine pancreatic juice sampling for cytological assessment under endoscopic retrograde pancreatography (ERP) for indolent BD-IPMN is not recommended in Fukuoka guidelines 2012, because ERP has a risk of lethal pancreatitis and sensitivity of pancreatic juice cytology to detect malignant IPMN is not so high, ranging from 10 % to50 % (3, 27, 32, 34). On the other hand, we have recently reported that ERP / pancreatic juice cytology has an important role to detect PDAC concomitant with IPMN which cannot be detected by other imaging modalities (15). In addition, Hirono et al. have reported that CEA level in pancreatic juice over 30ng/mL in addition to the size of mural nodule over 5mm is a good predictor for malignant BD-IPMNs, of which positive and negative predictive values are 100% and 96.3%, respectively (4). In MD-IPMNs, peroral pancreatoscopy under ERP is often useful to evaluate the intraductal spread of the tumor and thus to determine the adequate resection line during operation (13). Recent advancement of endoscopic tools allows us to perform biopsy for the target lesion in the pancreatic duct under the direct visualization using peroral pancreatoscopy (13).
The role of EUS-guided fine needle aspiration (FNA) cytology remains controversial because evaluation of the fluid sample to detect malignant IPMN has not been established and there is a risk of peritoneal seeding of the neoplastic cells (5, 27). Assessment of cystic fluid obtained by EUS-FNA can determine whether the cystic fluid is mucinous or not while the measurement of the CEA level cannot distinguish IPMN from mucinous cystic neoplasm at this time (27).
6. Pathological Aspects
Pathological diagnosis of IPMN is made according to the World Health Organization (WHO) classification 2010; low grade dysplasia (LGD), intermediate grade dysplasia (IGD), high grade dysplasia (HGD), and invasive carcinoma (22). One of the controversial issues is whether HGD should be included as a malignant entity or not. We have recently reported the patients having recurrent distant metastasis after resection of IPMN with high grade dysplasia, and thus consider HGD as malignant (25).
Another aspect is pathological subtypes; gastric, intestinal, pancreatobiliary, and oncocytic (Figure 5). Among them, the intestinal subtype has the characteristics of MUC2 positively in the cytoplasm of the neoplastic cells, while the other subtypes are negative for MUC2 expression; thus, IPMN can be also classified as intestinal and non-intestinal subtype (1).
7. Malignancy Predictors, Surgical Indication, and Surveillance Protocol
The prevalence of malignant and invasive MD-IPMN is 61.6 % and 43.1%, respectively, and thus, all the cases of MD-IPMN are basically indication for resection because of its high prevalence of malignancy (27). On the other hand, resection criteria in BD-IPMN remain controversial.

Figure 5. Pathological subtype of intraductal papillary mucinous neoplasm of the pancreas. (A) Gastric type with low grade dysplasia (x 200). (B) Intestinal type with an associated invasive carcinoma (x 200). (C) Pancreatobiliary type with an associated invasive carcinoma (x 200). (D) Oncocytic type with high grade dysplasia (x 200).
Fukuoka guidelines suggest unique issues to stratify the clinical and radiological findings of IPMN into 3 categories; namely, “high-risk stigmata”, “worrisome features”, and “low risk”, in terms of the prediction for malignant IPMN, and recommend the management strategy of IPMNs according to this stratification (Figure 6) (27). “High risk stigmata” include (1) obstructive jaundice in a patient with cystic lesion of the head of the pancreas, (2) enhancing solid component within cyst, (3) MPD > / = 10mm in size. Surgical resection should be considered in patients with one or more findings of “high risk stigmata”, if clinically appropriate.
On the other hand, worrisome features include (1) presence or history of pancreatitis, (2) cyst > / = 3cm in size, (3) thickened / enhancing cystic wall, (4) MPD size 5–9mm, (5) non-enhancing mural nodule, (6) abrupt change in caliber of MPD with distal atrophy, and (7) lymphadenopathy (27). If these findings are observed in CT and / or MRI, then EUS should be performed. If the following findings are detected by EUS, then surgical resection should be considered; (a) definite mural nodule, (b) main duct features suspicious for involvement, ( c) cytological result for suspicious or positive for malignancy (27). If EUS finding is inconclusive, then close surveillance should be recommended, while resection should be considered in young fit patients. The patients without any findings of high risk stigmata or worrisome features will be surveyed without resection in a schedule according to the size of the cyst. The Fukuoka guidelines also suggest that MPD diameter of 5-9 mm in MD-IPMN should be considered as one of the worrisome features, with a recommendation of the detailed evaluation but no immediate resection (27).
We have recently reported that the prevalence of malignant IPMN in patients having “high-risk stigmata”, worrisome feature”, and no risk factor were 61%, 26%, and 0%, respectively, which sound the reasonable percentage, and thus the stratification of Fukuoka guidelines seems to be adequate (16, 27).
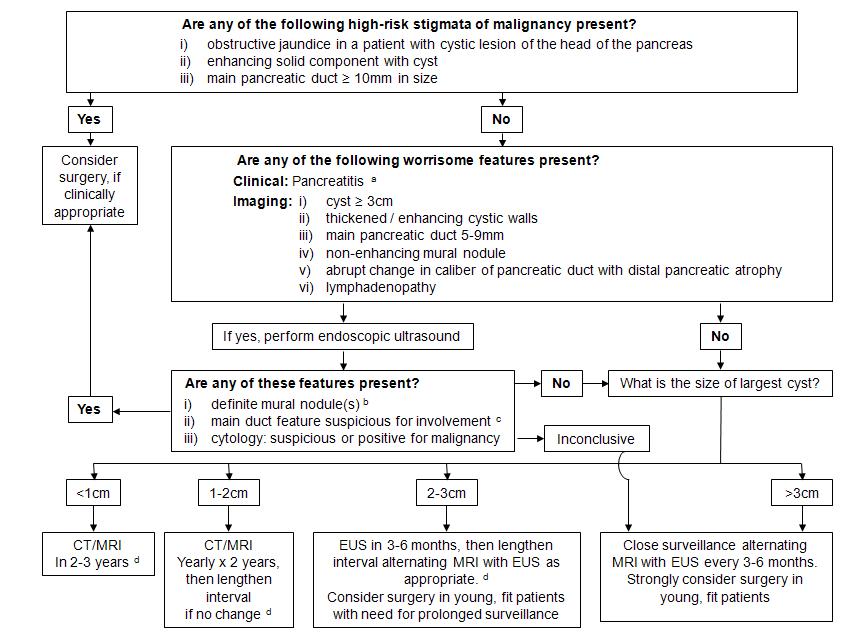
Figure 6. Algorism for the management of branch duct intraductal papillary mucinous neoplasms (BD-IPMNs) of the pancreas. (Cited from reference No. 27 with permission of the publisher)
a. Pancreatitis may be an indication for surgery for relief of symptoms.
b. Differential diagnosis includes mucin. Mucin can move with change in patient position, may be dislodged on cyst lavage and does not have Doppler flow. Features of true tumor nodule include lack of mobility, presence of Doppler flow and FNA of nodule showing tumor tissue.
c. Presence of any one of thickened walls, intraductal mucin or mural nodule is suggestive of main duct involvement. In their absence main duct involvement is inconclusive.
d. Studies from Japan suggest that on follow-up of subjects with suspected BD-IPMN, there is increased incidence of pancreatic ductal adenocarcinoma unrelated to malignant transformation of the BD-IPMN(s) being followed. However, it is unclear if imaging surveillance can detect early ductal adenocarcinoma, and if so, at what interval surveillance imaging should be performed.
In addition, the prevalence of malignant IPMNs, invasive carcinoma, and lymph node metastasis in patients with “high risk stigmata” were 80%, 55%, and 20%, respectively, and those values significantly increased in a stepwise manner according to the number of factors (2).
8. Natural history of BD-IPMNs Observed without Resection
Morphological changes of BD-IPMNs observed without resection such as an increase in the cyst size, dilation of MPD, appearance or an increase in size of mural nodule were observed in 27.4% of the 1,293 patients during surveillance period of 2.6 to 8.1 years in 12 articles (10). Among them, 9.9% of the patients underwent resection, demonstrating histologically malignant IPMNs in 27.3%, and thus, malignant transformation was observed in 2.7% of BD-IPMNs during surveillance period without resection (10). In addition, development of concomitant PDAC was observed in 2.8% of the study population (Figure 1) (10).
9. Operation
Considering the high prevalence of malignancy, MD-IPMN should be treated by pancreatectomy with regional lymph node dissection. The region of resection is determined based on the distribution of mural nodules. Mere dilation of the MPD without any mural nodules should not immediately resected and very careful examination and observation if necessary are required to distinguish chronic pancreatitis. During partial pancreatectomy for MD-IPMN, the margin status of MPD should be checked by frozen section because MD-IPMN has tendency to spread widely along the MPD (19). If the result shows HGD or invasive carcinoma, then additional resection should be performed to obtain a negative surgical margin (27). Although the effect of the presence of LGD or IGD at surgical margin on outcome remains controversial, additional resection is not recommended at this time in Fukuoka guidelines (27).
BD-IPMNs highly suspicious of malignancy should be resected by standard pancreatectomy with regional lymph node dissection, while those with low risk for malignancy can be resected by organ-preserving pancreatectomy including laparoscopic procedure (27). In multiple BD-IPMNs (25 to 41% of the cases), only significant lesions should be resected, and those without any malignancy predictors can be left without resection in the remnant pancreas (27, 11). During partial pancreatectomy, intraoperative irrigation cytology of the MPD in the remnant pancreas as well as frozen section pathology of the cut margin are often useful to detect unexpected PDACs which are not detected by preoperative imaging such as CT, MRI, and EUS (12).
10. Postoperative Surveillance
Surveillance protocol after resection of BD-IPMNs is determined based on the following factors; (1) pathological grade of resected BD-IPMNs, (2) pancreatic margin status after partial pancreatectomy, (3) presence of the residual lesions left without resection in the remnant pancreas, (4) presence of concomitant PDACs at the time of operation, (5) the possibility of metachronous occurrence of BD-IPMNs, and (6) the possible development of concomitant PDACs in the remnant pancreas (18). The yearly risk of concomitant PDAC development is reported to be 0.7 to 0.9% in the patients with BD-IPMNs, and thus Fukuoka guidelines suggest that CT or MRCP at 6 months intervals is appropriate for surveillance after resection of BD-IPMNs, even though the resected IPMN is benign with negative surgical margin (27). Surveillance with shorter interval should be considered in patients who underwent resection of invasive IPMNs, who had positive surgical margin status, or who have significant clinical signs to suspect the progression or new development of the disease (18). One example of a surveillance protocol after resection of BD-IPMN is presented in Figure 7.
On the other hand, surveillance schedule after resection of MD-IPMNs is determined based on pathological grade and surgical margin status (17). Prognosis after resection of invasive IPMNs is better than that of conventional pancreatic ductal adenocarcinomas (PDACs) in the matched status of T1 or N0, or in the subtype of colloid carcinoma, while it is not different from that of PDACs in the other conditions (T2 to T4, N1, or other subtypes of carcinoma) (20, 22). Thus, the patients with invasive MD-IPMNs should be surveyed according to the protocol of the conventional PDACs.

Figure 7. One example of surveillance protocol after resection of branch duct intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. (Cited from reference No. 17 with permission of the publisher)
In non-malignant IPMNs (LGD to IGD), if there is no residual lesion in the remnant pancreas with negative surgical margin for neoplastic cells, then the patients might be surveyed at 2 and 5 years after operation to check the development of new lesions in the remnant pancreas (17). In the patients having positive surgical margin of LGD to IGD, the surveillance of twice a year using physical examination and MRCP might be suitable, although there has been no evidence regarding the effect of this protocol (17). On the other hand, it remains unclear whether this surveillance protocol of twice a year would be also applied to the patients after resection for non-invasive carcinoma (HGD). If there are some clinical signs to suspect the progression of the diseases in such patients, then surveillance with shorter interval is recommended (17). One example of a surveillance protocol after resection of MD-IPMN is present in Figure 8.
We have recently demonstrated that MD-IPMNs often have a development of monoclonal skip lesion in the remnant pancreas, even after a partial pancreatectomy with surgical margin negative for neoplastic cells; however, additional resection of the remnant pancreas led to a favorable prognosis, and thus prophylactic total pancreatectomy for MD-IPMN at the time of the initial operation is not recommended (25). In addition, we have also experienced 3 patients having PDAC concomitant with MD-IPMN, and careful attention should be also paid to the possible occurrence of concomitant PDAC in patients with MD-IPMN as well as BD-IPMN (25).
11. Extrapancreatic Malignancy
Development of extrapancreatic malignancy is also reported in patients with MD-IPMNs as well as BD-IPMNs, the incidence being 10 to 45%, although etiology connecting IPMNs and the secondary neoplasms has not been known (21, 24, 33). The frequent sites of extrapancreatic malignancy in patients with IPMN are breast, prostate, and colorectum in Western countries, while stomach in Asian countries including Japan (21, 33). Thus, careful attention should be also paid to the extrapancreatic malignancy at the initial assessment and during surveillance of IPMN, although there is no recommended screening modalities to detect extrapancreatic neoplasms.

Figure 8. One example of surveillance protocol after resection of main duct intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. (Cited from reference No. 18 with reprint permission by courtesy of the publisher)
12. References
- Adsay V, Kloppel G, Fukushima N, et al. Intraductal papillary-mucinous neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al, editors. World Health Organization classification of tumors, pathology and genetics of tumors of the digestive system. Lyon, France: IARC Press; p.304-313, 2010.
- Aso T, Ohtsuka T, Matsunaga T, et al. "High risk stigmata" of the 2012 International Consensus Guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas, 2014. PMID: 25036910
- Hibi Y, Fukushima N, Tsuchida A, et al. Pancreatic juice cytology and subclassification of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 34: 197-204, 2007. PMID: 17312458
- Hirono S, Tani M, Kawai M, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy of branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 255: 517-522, 2012. PMID: 22301608
- Hirooka Y, Goto H, Itoh A, et al. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol 18: 1323-1327, 2003. PMID: 14535994
- Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 251:70-75, 2010. PMID: 200009749
- Ishigami K.CT and MRI/MRCP. In: Tanaka M (ed): Intraductal Papillary Mucinous Neoplasm of the Pancreas. Chapter 5, Pp. 45-66, Springer, Tokyo, 2014.
- Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 46: 22-29, 2014. PMID: 24218310
- Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in Japan. Pancreas 40: 364-370, 2011. PMID: 21289527
- Maguchi H, Tanno S. Natural history and malignant transformation of branch duct IPMN. In: Tanaka M, editor: Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pp. 19-26, Springer, Tokyo, 2014.
- Mori Y, Ohtsuka T, Kono H, et al. Management strategy for multifocal branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas 41; 1008-1012, 2012. PMID: 22850621
- Mori Y, Ohtsuka T, Tamura K, et al. Intraoperative irrigation cytology of the remnant pancreas to detect remnant distinct pancreatic ductal adenocarcinoma in patients with intraductal papillary mucinous neoplasm undergoing partial pancreatectomy. Surgery 155(1); 67-73, 2014. PMID: 24183345
- Nagayoshi Y, Aso T, Ohtsuka T, et al. Peroral pancreatoscopy using the SpyGlass system for the assessment of intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci 21: 410-417, 2014. PMID: 24123930
- Ohhashi K, Murakami Y, Maruyama M, et al. Four cases of mucous secreting pancreatic cancer. Prog Digest Endosc 20: 348-351, 1982.
- Ohtsuka T, Ideno N, Aso T, et al. Role of endoscopic retrograde pancreatography for early detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci 20: 356-361, 2013. PMID: 22878836
- Ohtsuka T, Matsunaga T, Kimura H, et al. Role of pancreatic juice cytology in the preoperative management of intraductal papillary mucinous neoplasm of the pancreas in the era of International Consensus Guidelines 2012. World J Surg. PMID: 25037612
- Ohtsuka T, Tanaka M. Postoperative surveillance of main duct IPMN. In: Tanaka M, editor: Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pp. 181-188, Springer, Tokyo, 2014.
- Ohtsuka T, Tanaka M. Postoperative surveillance of branch duct IPMN. In: Tanaka M, editor: Intraductal Papillary Mucinous Neoplasm of the Pancreas. Chapter 15, Pp. 189-199, Springer, Tokyo, 2014.
- Okada K, Imaizumi T, Hirabayashi K, et al. The distance of the tumor spread in the main pancreatic duct of an intraductal papillary-mucinous neoplasm: where to resect and how to predict it. J Hepatobiliary Pancreat Sci 17: 516-522, 2010. PMID: 20714841
- Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 251: 470-476, 2010. PMID: 20142731
- Reid-Lombardo KM, Mathis KL, Wood CM, et al. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas. Implication for management. Ann Surg 251: 64-69, 2010. PMID: 19858708
- Sadakari Y, Ohuchida K, Nakata K, et al. Invasive carcinoma derived from non-intestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from intestinal type. Surgery 147: 812-817, 2010. PMID: 20060146
- Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 42: 1077-1084, 2010. PMID: 21120776
- Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol 94: 470-473, 1999. PMID: 10022648
- Tamura K, Ohtsuka T, Ideno N, et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of the recurrences in the remnant pancreas after resection: a retrospective review. Ann Surg259: 306-308, 2014. PMID: 23989056
- Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6; 17-32, 2006. PMID: 16327281
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12:183-197, 2012. PMID: 22687371
- Tanno S, Nakano Y, Koizumi K, et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 39: 36-40, 2010. PMID: 19745777
- Tsutsumi K, Ohtsuka T, Oda Y, et al. A history of acute pancreatitis in intraductal papillary mucinous neoplasms of the pancreas is a potential predictive factor for malignant papillary subtype. Pancreatology 10; 707-712, 2011. PMID: 21242711
- Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasms of the pancreas. Gut 57: 1561-1565, 2008. PMID: 18477671
- Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2: 484-490, 2002. PMID: 12378117
- Yamaguchi K, Nakamura M, Shirahane K, et al. Pancreatic juice cytology in IPMN of the pancreas. Pancreatology 5: 416-421, 2005. PMID: 15985766
- Yamagchi K. Development of extrapancreatic malignancy. In: Tanaka M, editor: Intraductal Papillary Mucinous Neoplasm of the Pancreas. Chapter 10, Pp. 129-135, Springer, Tokyo, 2014.
- Yamaguchi T, Shirai Y, Ishihara T, et al. Pancreatic juice cytology in the diagnosis of intraductal papillary mucinous neoplasm of the pancreas. Significance of sampling by peroral pancreatoscopy. Cancer 104: 2830-2836, 2005. PMID: 16287152.
Figure 1. Pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. Enhanced computed tomography shows irregular low density solid lesion in the pancreatic body (arrow head) and cystic lesion in the pancreatic tail (arrow), indicating ductal adenocarcinoma in the pancreas body concomitant with intraductal papillary mucinous neoplasm in the pancreatic tail.


