Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2020.02
| Attachment | Size |
|---|---|
| 1.53 MB |
Abstract:
Intermediate filament keratins are cytoskeletal components in epithelial tissues, including the exocrine pancreas. Keratin (K) intermediate filaments are highly conserved proteins that are expressed from early developmental stages up to the differentiated epithelial cells in adult individuals. A multitude of specific cellular functions have been identified for keratins expressed in simple epithelia, such as the pancreas, liver, lung and intestine. These functions vary, depending on the cell- and tissue type, as well as the developmental stage and changes in the cellular environment. Keratins are composed of dynamic, post-translationally regulated cytoplasmic filaments built up of obligate heteropolymers of type I and a type II keratins. In simple epithelia, the main keratins are type II K8 and K7, and type I K18, K19 and K20.
The simple epithelium of the exocrine pancreas is responsible for the secretion of digestive enzymes into the duodenum. The exocrine pancreas comprises more than 85% of the pancreatic mass and consists of acinar and ductal cells. The acinar cells express mainly K8 and K18, which assemble into both cytoplasmic and apicolateral filaments, as well as minor levels of K19 and K20, which are confined to the apicolateral regions under basal conditions. Pancreatic duct cells express mainly K19 (type I) and K7 (type II).
Pancreatic keratins respond quickly to cell stress by keratin phosphorylation and filament breakdown followed by keratin upregulation, de novo filament formation and remodeling during the recovery phase in experimental exocrine pancreatic injury models. However, despite these dynamic stress responses, mutations or genetic deletion of K8 and K18 in humans or mouse models, only have modest effects on exocrine pancreatic health and stress tolerance. This is different from other simple epithelial tissues – most notably the liver – where K8, K18 and K19 mutations or deletions cause clear pathological outcomes. In contrast, overexpression of K8/K18 leads to pathological outcomes in the exocrine pancreas but not in the liver. These seemingly antagonistic outcomes in two cell types that have similar keratin expression patterns, underscores the versatile and tissue-specific function of keratins. The biological reasons underlying the different susceptibility of the exocrine acinar cells to keratin deficiencies, compared to other simple epithelial cells is not fully understood, but may, in part, be due to the increased levels of Regeneration protein-II observed in the pancreas in several K8/K18 deficient mouse models.
I. Introduction – Cytoskeletal Intermediate filaments
The cytoskeleton is an organized network of proteins present in all cells. In eukaryotic cells this network consists of three main filament systems: microtubules, microfilaments and intermediate filaments (IFs). Among the main cytoskeletal filament groups, IFs are, as their name suggests, intermediate in size (IF filaments are, as their name suggests, intermediate in size, measuring 10-12 nm in diameter; microfilaments are the smallest (6 nm) and microtubules the largest (25 nm) (26). IFs comprise a large and diverse group of proteins that are ubiquitously expressed. They are divided into 6 different types, where type I and type II comprise different keratins, type III includes the muscle IFs desmin and vimentin expressed in mesenchymal cells, type IV neurofilaments, nestin and α-internexin expressed e.g. in nerve cells, as well as the muscle cell IF, synemin. Type V comprises the lamins, which are nuclear IFs found in all nucleated cells, including all epithelial cells (9). Finally, group VI comprise the highly specialized IFs phakinin and filensin, expressed only in the eye lens. The different types of IFs are typically expressed in a highly cell and tissue specific and/or in a developmentally specific manner (18, 27).
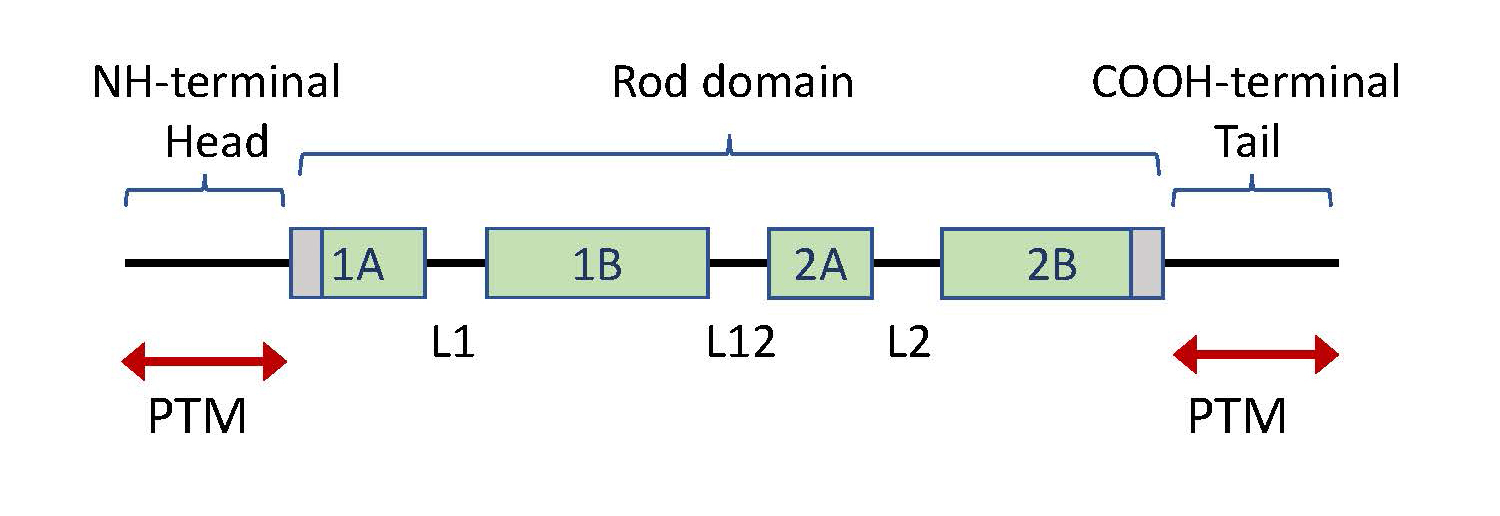
Keratins, as well as all other IF proteins, have a basic structure of a central coil-coil α-helical rod domain, flanked by an N-terminal head domain and a C-terminal tail domain of variable length (Figure 1). Two IF molecules dimerize to form tetramers, which are the building blocks of these mechanically strong, yet flexible filaments. The IF assembly does not require ATP and IF filaments are non-polar, in contrast to microfilaments and microtubules (27, 46, 56). Keratins, as well as other IFs, are dynamically regulated through various post-translational modifications (PTM), including phosphorylation, O-linked glycosylation, ubiquitination, sumoylation, acetylation, and transamidation (58).
Figure 1. Keratin protein structure. All keratins are composed of a central helical rod domain, flanked by a head domain at the amino terminal (N-terminal) and a tail domain at the carboxy-terminal (C-terminal) of the protein. The rod domain is segmented into 4 parts; 1A, 1B, 2A and 2B, interlinked by short linker regions (L1, L12, L2). The peripheral sites of the rod domain (depicted in grey) contain highly conserved regions in keratins. The head and tail consist of non-helical segments, which contain most of the sites for post-translational modifications (PTMs), including multiple phosphorylation sites (47, 69).
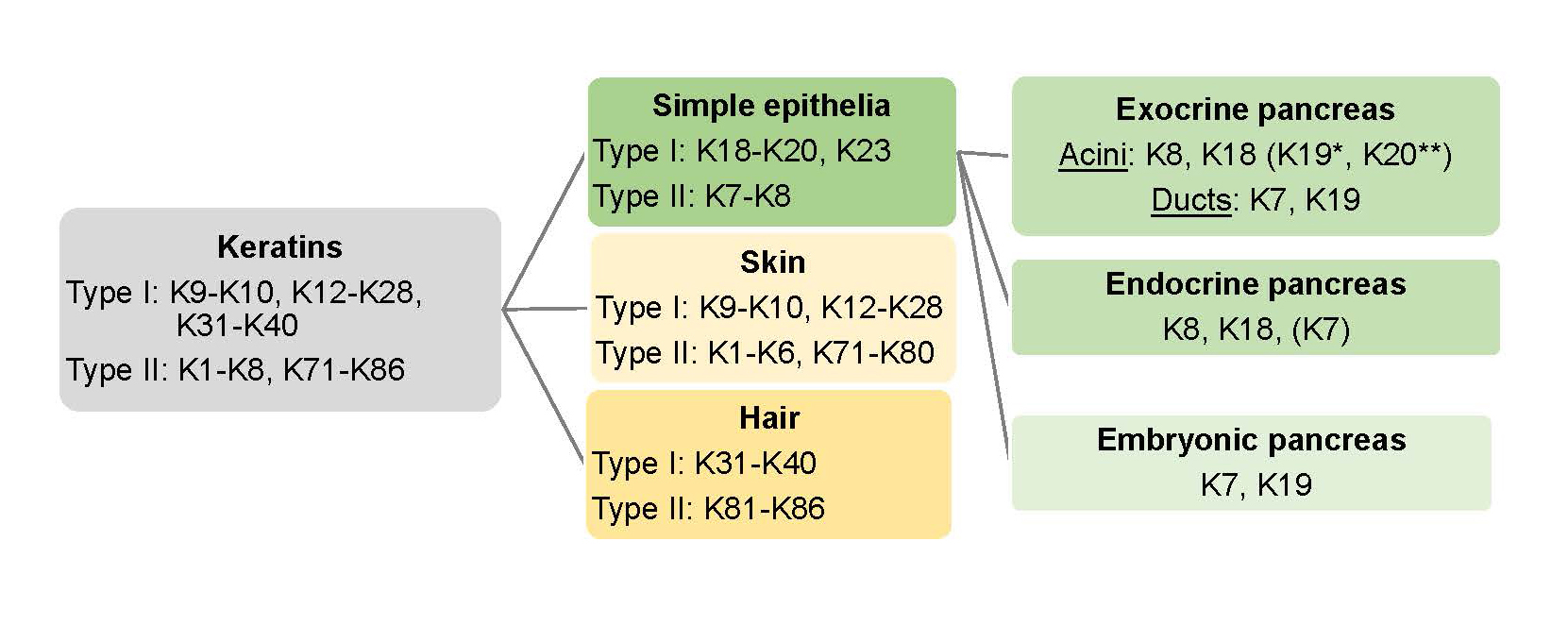
The keratin family includes 54 different functional keratin genes and proteins that are divided into two generic groups: type I, acidic keratins, and type II, neutral or basic keratins. Type I and type II keratins form obligate heteropolymers consisting of at least one of each type. Keratins have a molecular weight in the range of 44-66 kD and constitute the main cytosolic IFs in all epithelial cells such as pancreatic acinar, duct and endocrine islet cells. The different types of keratins are further expressed in a cell- and tissue-specific manner (35, 56) (Figure 2). The human type I and type II keratin genes are clustered on the human chromosome 17q21.2 and 12, respectively, with the exception of type I K18, which is located adjacent to type II K8 on chromosome 12q13.13 (28). K8 and K18 are thought to be the closest descendants to an ‘ancestral keratin pair’ and the adjacent location of these two keratins may reflect an early divergence within the keratin gene family (43, 77). K8/K18 is also the earliest keratin pair expressed in embryogenesis (28).
Simple layered epithelial cells, found e.g. in the intestine, lung, liver and pancreas, predominantly express the type II keratin, K8, (and to a lesser extent K7) and type I keratins K18, K19, K20 and, in a few epithelial cells, K23. The specific type I and type II expression pairs differ between different organs, as discussed below. Overall, K8/K18 is the predominant pair in the liver and pancreas, while K8/K19 heteropolymers predominate in intestinal epithelial cells. However, in hepatocytes, K8/K18 is the only keratin pair, whereas in the pancreatic acinar cells and in intestinal cells, K8 pairs with both K18, K19 and K20 (69, 85).
Figure 2. Keratin expression in epithelial tissues and the pancreas. Intermediate filament type I and type II keratin (gray box) proteins are divide in skin keratins (light yellow box), hair keratins (yellow box) and simple epithelial keratins (green box). The main keratins in adult human exocrine pancreas acinar cells are K8, K18, with the addition of K19* in the centroacinar cells. K19 and K7 are expressed in mammalian exocrine ducts (rat ducts also express K20). Mouse acinar cells also express mainly K8 and K18, but in addition lower levels of K19* and K20** near the apicolateral membranes. The endocrine pancreatic cells (in mouse) express K8 and K18, and lower levels of K7. K7 and K19 are found in the embryonic pancreas.
Of all simple epithelial organs, liver is the most affected by keratin abnormalities (33). This disease susceptibility is probably due to hepatocytes expressing only K8 and K18 (35, 63). Several liver disease-associated mutations of K8, K18 and K19 have been identified in humans (45, 46, 69). Mouse models expressing these mutations often phenocopy the disease (33, 46) and are therefore important for studies relating to keratinopathies. Since K8 is the main type II keratin in most simple epithelia, the K8 null mouse, as well as mice overexpressing wild-type K8 or disease-related K8 or K18 mutations, present valuable tools for studying the roles of keratins in simple epithelial organs. The liver and colon are the organs most affected by these keratin deficiencies and they are also the most studied in this context (46). The exocrine pancreas is less affected by K8 deletion; the K8 null exocrine pancreas appears, in fact, to be modestly protected from experimental pancreatitis-induced injury (68). Experimental K8 and K18 overexpression, conversely, conveys age-associated atrophic changes to the exocrine pancreas, yet does not result in any known pathological anomalies in the liver (71). In this review, the expression and regulation of keratins in the exocrine pancreas during basal conditions and pancreatic injury as well as keratin-dependent regulation of cell stress-response proteins will be reviewed. Further, the implications of keratin defects on different types of pancreatic injury and the changes in keratin expression in pancreatitis and pancreatic cancer will be discussed.
II. Intermediate Filament Keratin Function, Regulation and Disease Association
IFs, including keratins, were first recognized as structural components of cells, serving mainly as static mechanical support for the cells. Further investigations into the structure and function of keratins nevertheless revealed that they are, in fact, highly dynamic structures that assemble and disassemble very quickly, e.g. by means of post-translational regulation in response to various stimuli (59). Phosphorylation is the most frequently occurring -and also the most studied - PTM in keratins. The PTM sites are mainly located on the head- and tail domains of keratins and these domains typically contain multiple phosphorylation sites (Figure 1). Keratin PTMs enable rapid and dynamic regulation of the keratin filament solubility, as well as network assembly and organization (47). Keratin PTMs, moreover, regulate the association of keratins with multiple essential cellular proteins, such as the cytolinker protein plectin, 14-3-3 adapter protein as well as several protein kinases and phosphatases (6, 39, 47). Keratins attach to the desmosomes and hemidesmosomes through cytokinker proteins, thus forming a cytosolic network that extends from the cell membrane to the nucleus, providing a flexible yet mechanically stable cellular reinforcement (76). Keratins are, as a consequence of their interaction with multiple cellular proteins, involved in diverse cell physiological processes, in addition to providing dynamically adaptable mechanistic reinforcement in the cell (79). The multitude of different proteins that interact with keratins, many of which are essential cell-signaling mediators hence imply a central regulatory role for epithelial cell keratins in intracellular organization, cell signaling, maintaining cell polarity, regulating translation and targeting proteins and organelles in the cell (2, 19, 30, 41, 46, 48, 57, 59, 74).
Keratins also serve a vital role in the protection from both mechanical and non-mechanical cell stress. A strong cell specific upregulation of keratin protein and / or mRNA takes place after different types of injury. This upregulation, that often occurs in the regenerative phase after injury, can be observed, for example, in response to skin damage, liver injury and to chemically induced pancreatic injury, and may include de novo expression of stress-induced keratins (34), as discussed below. The upregulation of keratins in response to cell stress provides mechanical reinforcement for the cells but may also regulate cell responses on a more intricate level through keratin interactions with cellular pathways that determine cell survival, apoptosis or regeneration (50).
Given the multiple functions for keratin IFs, it is hardly surprising that keratin deficiencies or mutations play a role in several human diseases. Mutations in skin keratins cause several different diseases including epidermolysis bullosa simplex, and mutations in simple epithelial keratins increase the susceptibility to various liver diseases (45). Research work based on animal models further links keratin abnormalities with skin and nail disorders, as well as dysfunctions in hair and liver (45). K18 null mice develop age-dependent pathological hepatocyte anomalies, resembling chronic cirrhosis, while K8 ablation causes liver insufficiencies (53, 72, 73) as well as a microbiota-dependent colitis (22, 23). The K8 null colitis is accompanied by deficiencies in ion-transport and energy metabolism, a Notch1-associated cell fate shifts towards a more secretory cell type, increased inflammasome signaling, as well as increased susceptibility to colorectal cancer (4, 25, 36, 42). In the murine thymus, K8 deletion causes increased apoptosis (44). In the endocrine pancreas, we have demonstrated mislocalization of the β-cell glucose transporter 2 (GLUT2), defects in mitochondrial morphology and function as well as decreased insulin levels associated with abnormal insulin vesicle morphology in K8 null mice (2, 57). Moreover, mice with reduced K8 expression (K8 heterozygote null mice) show increased susceptibility to experimental type I diabetes (3). In many cases, however, the keratin mutation does not in itself cause a disease, but it constitutes a risk factor and renders the affected individual more vulnerable to certain conditions (46, 69). These indirect disease associations are not always easy to verify, as the keratin mutation may be one of many factors contributing to a disease but may nevertheless be an important susceptibility factor for diseases/ dysfunctions of epithelial cells and tissues.
III. Keratin Expression in The Exocrine Pancreas in Humans and Murine Models
The exocrine pancreas consists of acinar cells that secrete digestive enzymes, and a network of ducts that transport these enzymes from the pancreas to the small intestine (51). The acinar cells are pyramidal shaped simple epithelial cells arranged in acini, whereas the duct cells are simple squamous or cuboidal type epithelial cells (51). Keratins make up the IFs of the pancreas and comprise 0.3% of the total pancreatic proteins (84). The pattern of keratin expression differs between different species and developmental stages. The subcellular localization of the different keratins in the pancreas is moreover highly orchestrated and molded by the cell-specific conditions.
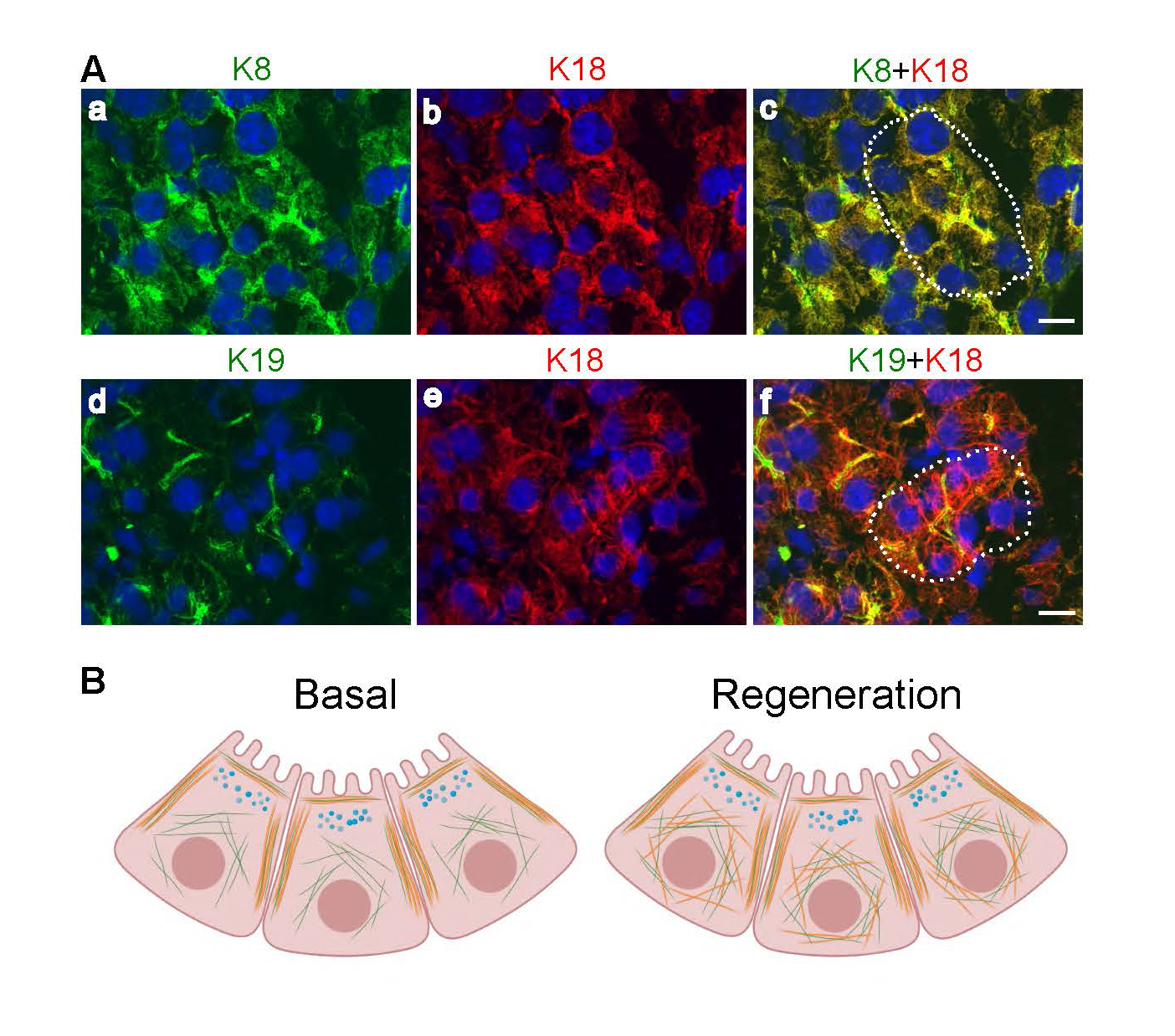
Under basal circumstances, adult mouse acinar cells express K8 and K18 with minor levels of K19 and K20 (Figure 2). K8 and K18 form cytoplasmic filaments throughout the acinar cell, including prominent keratin bundles (known as apicolateral filaments) running along the apical and lateral domains, in parallel to the F-actin layer closest to the cell membrane (68). In contrast, K19 and K20 are only observed apicolaterally under basal circumstances (Figure 3) (68, 70, 84). K19 and K7 are the main keratins of exocrine pancreatic duct cells in mice (68), and K19, K7 as well as K20, in rats.
Figure 3.Keratin expression in mouse exocrine pancreatic acinar cells. A. K8 (a, green) and K18 (b, red) form cytoplasmic heterodimeric filaments (merged image of K8/K18 is shown in c) in acinar cells whereas K19 (d, green), here co-stained with K18 (e, red; merged image of K19/K18 in f), is observed apicolaterally. Nuclei are shown in blue and an acinus in c and f with basally located nuclei are outlined with a dotted line. Scale bar = 20 μm B. The schematic illustration shows acinar cell cytoplasmic and apicolateral K8/K18 (green) and apicolateral K19 and K20 (orange) filament localization under basal conditions (left), and the apicolateral as well as de novo K19/K20 cytoplasmic filament localization during regeneration after acinar cell injury (right).
Outside the scope of this review, the mouse endocrine pancreas, consisting of islets of Langerhans, also expresses mainly K8 and K18, but also some K7, which like K8, forms heteropolymers with K18 (2). Additionally, K20 expression has been reported in neonatal rat endocrine pancreas (8, 78).
In humans, K7 and K19 are expressed in all epithelial cells during fetal development. Postnatally, differentiated human acinar cells only express K8 and K18, while K7 and K19 expression is retained in the pancreatic duct cells (7) and K19 in centroacinar cells (68). K20, does not appear to be expressed under basal conditions in human pancreatic duct cells to any significant degree (8, 78).
The physiological importance of these subtle inter-species pancreatic cell differences in keratin expression is not known. However, given the universal expression of K7 and K19 in early fetal development, retention of K19 in rodent acinar cells may be indicative of a lower level of cellular differentiation. Furthermore, K20 expression in the neonatal rat endocrine pancreatic cells is associated with cell proliferation (7) and increased expression of mouse K19 and K20, with acinar cell regeneration (see below Section VII). It may hence be speculated that the presence of K7, K19 and K20 expression in rodent pancreas reflects a lower level of cellular differentiation and perhaps higher degree of plasticity and regenerative capacity. This hypothesis is in line with the observations that pancreatic regeneration after injury, obesity and during pregnancy is significantly higher in rodent than in human pancreatic cells (11).
IV. The Effects of Experimental Keratin Mutations, Deletion or Overexpression, in The Exocrine Pancreas Under Basal Conditions
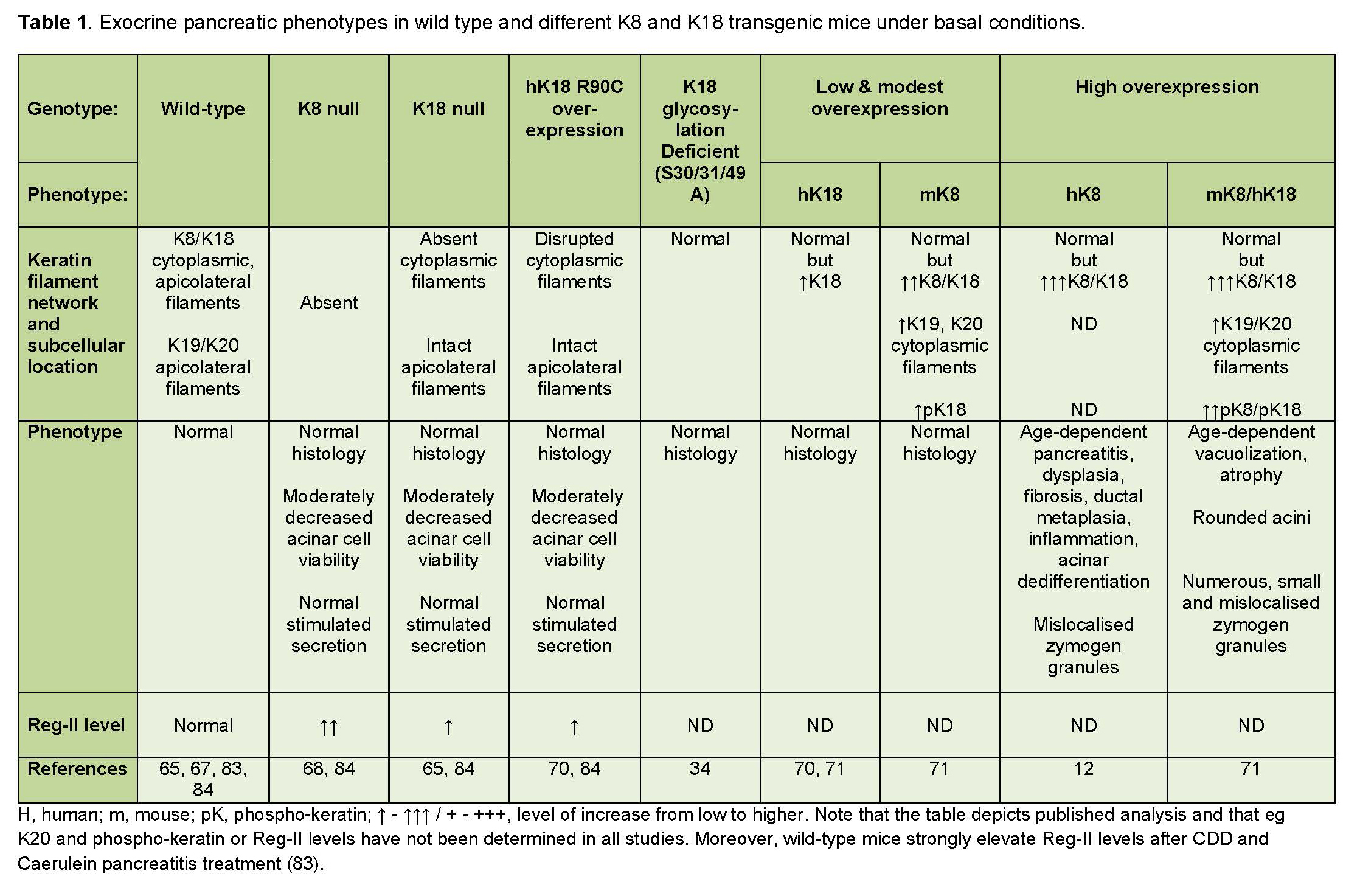
The exocrine pancreas has a strikingly high tolerance to keratin absence or mutations, when compared to similar keratin deficiencies in the liver, which also predominantly expresses K8 and K18. The subject matter deserves consideration since these findings challenge a simplistic view of keratins as static stress protectors in all cells and bids for a more scrutinizing analysis of the mechanisms underlying the stress protein functions of keratins. Several transgenic mouse models that either lack or overexpress wild-type keratins, or that overexpress specific human keratin mutations have been used to explore the role of keratins in the exocrine pancreas (summarized in Table 1).
Table 1. Basal pancreatic phenotypes of keratin transgenic mice.
A. K8 and K18 null mice
The only type II keratin expressed in acinar cells is K8. Since keratins are obligate heteropolymeric proteins and require both a type I and type II keratin to form stable filaments, it is expected that acinar cells in mice lacking K8, are entirely void of keratins. Indeed, the absence of both K8 and K18 has been demonstrated by immunofluorescence labelling and immune-electron microscopic analysis in K8 null mice. In contrast, K18 null mice still express K8, since K8 forms heteropolymers with K19 in the absence of K18. However, under basal circumstances, K8/K19 keratin filaments are located solely to the apicolateral region of the acinar cells in K18 null mice, as K19 cannot compensate for cytoplasmic K18 filament formation under basal conditions. However, the histology of the K18 null pancreas is normal, apart from some poly-nuclear areas that bear a resemblance to the histological observations in the livers of K8 null and K18 null mice (68, 72). The secretory response upon stimulation by cholecystokinin octapeptide (CCK-8) is normal in K8 and K18 null mice, but the acini are moderately less viable than in wild-type mice (68). The modest phenotype changes in K18 null mouse pancreas may be explained, at least in part, by the existence of the K8/K19 heteropolymers. However, in K8 null mice, the resistance to injury probably comes down to other compensatory mechanisms (68). One such suggested mechanism is the upregulation of regulatory protein II (Reg-II), which occurs in the K8 null pancreas. This will be discussed in greater detail in sections V of this review.
B. Transgenic mice overexpressing wild-type keratins
In contrast to keratin deletion, keratin overexpression is well tolerated in the liver, while in the pancreas it is associated with various pathological changes (12, 71). In the study by Casanova and colleagues, which involved transgenic mice overexpressing human K8, severe age-dependent progressive abnormalities were observed in the exocrine pancreas, demonstrated by a 30% loss of pancreatic mass, dysplasia, fibrosis, ductal metaplasia, inflammation and dedifferentiation of acinar cells into duct cells (12). The study by Toivola et al. (2008) showed that the extent of pancreatic damage correlates with the level of keratin overexpression (71). This latter study used human K18 overexpressing mice where keratin levels were only minimally increased, mouse K8 overexpressing mice with moderately upregulated K8, K18, K19 and K20, as well as mice that overexpressed both mouse K8 and human K18 (K8/K18 overexpressors), leading to a substantial keratin upregulation (71). Though the effects of keratin overexpression were less severe in the study by Toivola and colleagues, similar acinar cell anomalies were observed as by Casanova et al, such as age-dependent atrophy and fatty vacuole formation - particularly in the mice with the highest levels of keratin overexpression. Hence, it is likely that the aggravated injury in the earlier study may have been due to a higher level of keratin upregulation (71).
Genetic overexpression of both K8 and K18 in pancreatic acinar cells changes the distribution and phosphorylation level of keratins and causes alterations in the quantity, size, and distribution of the amylase-containing zymogen granules in the acinar cells. The zymogen granules in K8/K18 overexpressing cells, as well as in human K8 overexpressing mice, were smaller in size, but more numerous than in wild-type mice (71). Moreover, the granules were not retained to their characteristic apical location but instead diffusely localized in the cytoplasm in K8/K18 overexpressors (12, 71). Thick K8/K18 bundles, visualized using electron microscopy, were located mainly around the apical lumen in wild-type acinar cells, but were frequently localized also to perinuclear and cytoplasmic locations and the phosphorylation level of keratins, which often corresponds with cell stress (47), was increased at K8 S79 and K18 S33 in K8/K18 overexpressors (71). Hence, overexpression of keratins in acinar cells appears to interfere with the intracellular organization of keratin and alter the exocrine function of the acinar cells. Interestingly, mice that overexpress a K18 S33A mutation (which inhibits serine 33 phosphorylation), display keratin filaments that are retracted from the basal and nuclear region and instead concentrated in the apical region of acinar cells. However, these mice do not display abnormal pancreatic histology or disease (32).
These studies of the exocrine pancreas, using keratin overexpressor mice, further highlight the interrelationship between type I and type II keratins. Since K18 overexpression caused a minimal increase in keratin levels compared to K8 overexpression, it is evident that the level of type II keratins has a more profound effect on overall keratin regulation than type I keratins in the exocrine pancreas. Moreover, as the exocrine pancreas contains more than one type I keratin (K18, K19, K20), a deficiency or upregulation of one of these may be compensated, to a certain extent, by a down- or upregulation of another type I keratin.
C. Transgenic mice overexpressing human diseases-related keratin mutations
The contribution of specific keratin mutations for increased susceptibility to injury or disease has been analyzed with the help of transgenic mouse models, expressing human keratin mutations. Many of these experimental models, including human K18 R90C mice (which have a mutation equivalent to K14 R125C, found in epidermolysis bullosa simplex patients) as well as human K8 G62C mice (expressing a common human keratin variant mutation in liver disease patients), display early-onset liver inflammation, necrosis and increased susceptibility to hepatotoxicity (5, 33, 73). The exocrine pancreas in these mice, on the contrary, appears far less affected by these mutations. Although the viability of the acini is somewhat compromised in K18 R90C mice displaying disrupted keratin filaments, these mice do not display increased pancreatic injury under basal circumstances (68). K18 R90C mice lack intact cytoplasmic keratin filaments (displaying only K8/K18 dots in the cytoplasm), but they express apicolateral filaments in acinar cells, since K8 polymerizes with K19 and K20 which are located apicolaterally under basal conditions. This is in contrast with K18 R90C mouse hepatocytes, in which apicolateral keratin filaments are scarcer. This difference between hepatocyte and pancreatic keratins have been suggested as one reason underlying the lesser disease susceptibility of the pancreas compared with the liver in the K18 R90C transgenic mouse model (68).
V. Keratins in Mouse and Cell Models for Pancreatic Injury
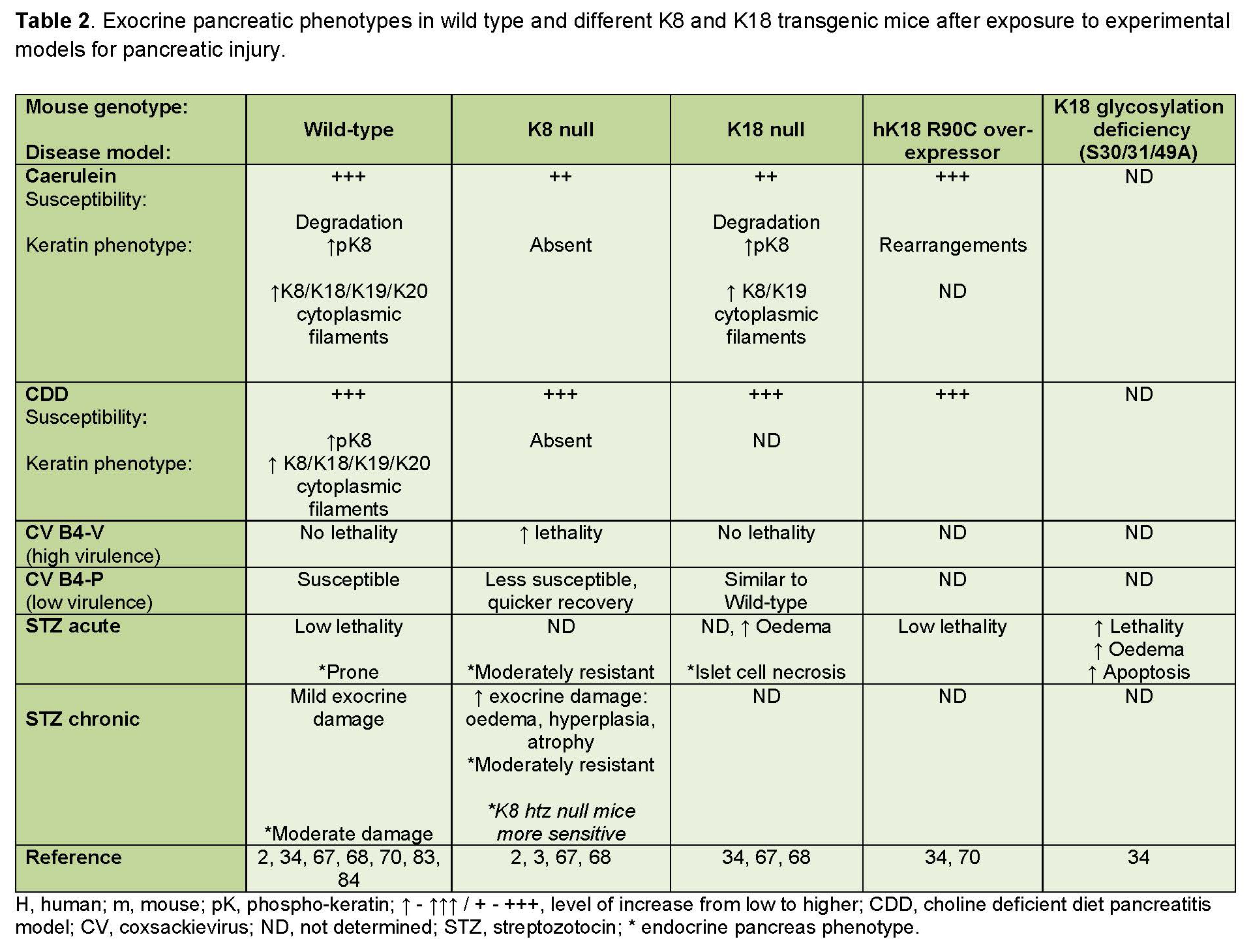
Keratin networks are dynamic and respond swiftly to changes in the cellular environment. Several studies have investigated the roles of keratins in the exocrine pancreas by subjecting wild-type and K8 or K18 deficient mice to experimental pancreatitis models, including the caerulein model and the choline-deficient diet model (81) (summarized in Table 2). Typically, in these conditions, keratins are first rapidly broken down upon acinar cell injury, but reform quickly and become highly upregulated during recovery. During the recovery process, keratins are also extensively phosphorylated, as is common in keratin stress-responses. This dynamic process has been described after caerulein-induced exocrine pancreatic injury, which causes a rapid disassembly of the acinar cell keratin network within 1 hour of induction of injury, followed by a reassembly of keratin cytoplasmic filaments in the early recovery phase 7 -24 hours after induction of injury. The cytoplasmic keratin network reforms during the recovery phase, and temporarily, strong de novo cytoplasmic filaments containing K19 and K20 become very prominent (Figure 3B), in addition to the K8/K18 cytoplasmic filaments (34, 68). This keratin upregulation is transcriptionally regulated since keratin mRNA levels also increase 48 hours after caerulein induction (84).
Table 2. Experimental pancreatic injury phenotypes in wild-type and keratin transgenic mice.
Ultimately, in the late recovery phase (within 5 days), retrieval of the normal, base-line filament network structure occurs, and K19 and K20 filaments repossess their exclusively apicolateral localization. A similar keratin upregulation on protein and mRNA level, accompanied by de novo cytoplasmic K19 and K20 filament formation, is seen 1-2 days after discontinuation of choline-deficient diet feeding, with keratin levels returning to baseline levels 7 days into the recovery phase (84). A similar transitory, recovery phase apical- to cytoplasmic localization shift also appears in caerulein-treated K18 null mice, in which the keratin network under basal circumstances is exclusively apicolateral in acinar cells. In this model as well, the filaments revert back to their apicolateral localization later in the recovery phase (68). It is still unknown what regulates the induction of this remarkable transient K19 and K20 cytoplasmic filament formation, but it is suggested that it plays an important role in the recovery of acinar cells after injury. The existence of K19 filaments in K18 null mouse acinar cells has been postulated as a possible reason for the high tolerance to experimentally induced pancreatic injury in these mice (68). If this is the case, it is possible that K18 mutations may be more detrimental for human acinar cells, which lack K19 filaments. Interestingly, the keratin upregulation appears to be specific to regeneration after pancreatitis, since generalized stress models such as heat or water-immersion, does not alter keratin expression levels (84). However, TGFβRII dominant negative mutant mice, which develop a severe chronic pancreatitis phenotype similar to human K8 overexpressing mice, show highly increased K8 and K18 levels in the pancreas (12). Keratin gene transcription in the pancreas has not been extensively studied, but the K19 gene in pancreatic duct cells is regulated by PDX1, GKLF/KL4 and SP1, indicating an association between K19 expression and the developmental stage in the pancreas (10, 16).
Interaction with the keratin-binding protein, epiplakin, contributes to the dynamics of pancreatic keratin remodeling after caerulein injury, as epiplakin-deficient mice display both a quicker keratin network breakdown after injury, as well as impaired filament rearrangement, as demonstrated by the accumulation of keratin aggregates at the most severe phase of the cell injury. These defects might be caused by excessive hyperphosphorylation in the absence of epiplakin (61, 80), emphasizing the role of dynamic keratin regulation in stress responses. In addition to the stress-induced keratin filament remodelling, keratins could also be involved in caerulein-induced inflammatory responses in the acinar cells, since caerulein-induced keratin upregulation is associated with nuclear factor-κB (NF-κB) activation (84).
The extensive rearrangement of keratin filaments in response to pancreatic injury in caerulein- and choline-deficient diet-induced pancreatitis, suggests a protective role for keratins in these injury models. Yet intriguingly, apart from minor differences observed in certain disease parameters, K8 null (which lack acinar cell keratins entirely), K18 null (which express only K8/K19 acinar cell filaments) and K18 R90C mice, are not overall more sensitive to choline deficient diet or caerulein-induced pancreatitis, compared with wild-type mice (68). In fact, even with more prominent vacuolization, the histological damage, in terms of inflammation and edema is slightly lower in K8 null and K18 null mice after caerulein, compared to wild-type mice (68). Moreover, no differences in pancreas histology, serum amylase or lipase levels were observed in K18 R90C mice compared with wild-type K18 overexpressing mice (70, 83). These seemingly near dispensable effects of keratin deficiencies in the exocrine pancreas are puzzling. However, some potential underlying reasons for the relative stress tolerance of the exocrine pancreas in keratin deficient mice can be extrapolated from existing animal studies. These include differences in the cellular localization of keratins in the acinar cells and hepatocytes, upregulation of cytoprotective regulatory proteins (discussed in section VI), and differences in the function of keratins in different organs (70, 83). Further, the importance of keratins in pancreatic stress may also depend on the type and duration of the injury. For example, in a study where K8 null and K18 null mice were challenged with coxsackie B virus-induced pancreatitis, the vulnerability of keratin deficient mice depended on the virulence of the coxsackie virus strain. When subjected to acute pancreatitis caused by the highly virulent coxsackie virus strain B4-V (CVB4-V), keratin null mice suffered significantly higher mortality compared with wild-type mice (40 % mortality in K8 null mice and 0 % in K8 wild-type mice). In contrast, K8 null recovered quicker than their wild-type counterparts from infection with a less virulent, B4-P coxsackie virus strain (CVB4-P). It has been proposed that this difference may come down to the effect of Reg-II-stimulated tissue regeneration, since Reg-II upregulation occurs after infection with CVB4-P virus as well as in the caerulein and choline-deficient diet pancreatitis models, but not CVB4-V in wild-type mice (67, 83). Interestingly, Reg-II is upregulated in the K8 null pancreas under basal conditions (83) and this upregulation may assist in the injury response to CVB4-P-induced pancreatitis, as will be discussed in section VI.
In addition to CVB4-V coxsackie virus-induced pancreatitis, keratin-deficient animals also appear to be more sensitive to exocrine pancreatic injury induced by streptozotocin (STZ). STZ is a commonly used toxin for inducing type I diabetes in experimental animals. This toxin is taken up by β-cells through the glucose-transporter 2 (GLUT2) and causes acute β-cell destruction if administered at high doses, and partial β-cell depletion and inflammation if administered at multiple low doses (2, 65). Interestingly, K8 null mice develop widespread exocrine pancreatic edema, atrophy, vacuolization and inflammation in response to the chronic stress induced by low-dose, STZ treatment; while wild-type animals display only modest exocrine damage after this treatment (2). Interestingly, K8 null β-cells are less sensitive to acute high-dose STZ (200 mg/kg of body weight), likely due to mislocalization of GLUT2, which is needed for the uptake of STZ into β-cells. Yet, transgenic mice expressing a human K18 glycosylation-preventing (K18-Gly(-)) mutation suffer severe exocrine pancreatic injury after the same high-dose STZ treatment (34). The STZ treatment in this study induced multi-organ failure in the glycosylation-deficient K18 mice, hence the toxic effects on the pancreas may have been exacerbated by the keratin mutation induced liver deficiency in these animals (34). Taken together, these results demonstrate that the function of keratins, and their importance in protecting from cellular injury in the pancreas, may be crucially dependent on the disease mechanisms of a particular experimental model, the duration of the injury and the type of keratin anomaly. Interestingly, in addition to keratin IFs, the type V intermediate filaments, nuclear lamins, may also contribute to pancreatic stress protection, since pancreas-specific conditional lamin A null mice show a spontaneous phenotype similar to chronic pancreatitis, and patients with mutations in lamin A have a higher risk of developing pancreatitis (17, 24).
Dynamic stress-induced regulation of keratins has also been described in acinar cancer cell cultures in vivo, under various experimental stress conditions. For example, K23 mRNA was highly induced by the histone deacetylase inhibitors sodium butyrate and trichostatin during differentiation in the pancreatic cancer cell line AsPC-1 (82), demonstrating the propensity for keratin de novo expression during stress conditions. In pancreatic carcinoma, PANC-1 cells, which express K8 and K18, caerulein treatment induced K8 S431 phosphorylation and a reorganization of the keratin filaments into a tight perinuclear network through activation of ERK and downregulation of PP2A and alpha 4, resulting in enhanced cell migration (52). Interestingly, similar K8 phosphorylation and perinuclear reorganization has also been observed in PANC-1 and A549 cells (human carcinomic alveolar cells) exposed to metastasis-enhancing bioactive lipid sphingosyl-phosphorylcholine, 12-Otetradecanoylphorbol-13-acetate (TPA), leukotriene B4 (LTB4), or shearing force (29, 52).
VI. Regenerating Protein II as a Cytoprotective Factor in Keratin-Deficient Exocrine Pancreas Models
The regenerating (Reg) gene was first isolated from islet β-cells in the pancreas (66). The protein encoded by this gene (now termed Reg-I) was found to have a significant stimulating effect on β-cells in the endocrine pancreas and was able to ameliorate diabetes in 90% pancreatectomized rats and in non-obese diabetic (NOD) mice (21). It was later discovered that the Reg genes constitute a multigene family consisting of three types of genes (Reg-I, II and III) that differ in expression pattern and functional characteristics, as reviewed in (1, 83). In the endocrine pancreas, Reg-I seems to play an important role in islet regeneration, while in the exocrine pancreas Reg-II is the predominant Reg-protein. However, Reg-II is substantially upregulated in the exocrine pancreas during recovery from caerulein or choline-deficient diet-induced pancreatitis (83), which indicates that it has an analogous function to that of Reg-I in the endocrine pancreas. Interestingly, in the caerulein model, Reg-II levels increase early on, while the K18 and K19 levels are at their highest later in the recovery phase, but in the choline-deficient model, Reg-II and K18 peak around the same time (83).
The unexpectedly high resistance to pancreatic damage in keratin deficient or mutant mice discussed above has encouraged analysis of compensatory factors in these mice that might explain why keratin deletion causes less damage in the exocrine pancreas compared to the liver, despite a similar keratin expression pattern. In a microarray analysis of K8 null mouse pancreas, several genes were indeed found up- or downregulated compared to wild-type control mice. Among the significantly upregulated genes were several members of the Reg-gene family, but particularly the Reg-II gene. Upregulation of Reg-II was also observed in other mice with keratin deficiencies, such as K18 null, K18 R90C transgenic mice, and phosphorylation deficient K18 S52A transgenic mice. However, this upregulation was not seen in K19 null mice, which express normal, cytoplasmic and apicolateral keratin filaments (83). It has thus been suggested that the resistance to pancreatitis in K8 null and K18 null mice likely comes down to a compensatory protective effect of the Reg-II upregulation. This compensatory mechanism could explain the greater resistance of K8 null mice to the moderate pancreatic injury caused by infection with CVB4-P, which is accompanied by anti-apoptotic and cell regenerative responses, as well as to the acute injury by CVB4-V infections, which causes an upregulation of genes that favor apoptosis, metaplasia and fibrosis (49, 68, 83).
The benefit of acinar cell Reg-II overexpression for recovery from experimental pancreatitis and diabetes has been questioned in a study by Li and colleagues (2010), since transgenic acinar cell-specific overexpression of Reg-II neither protected the mice from streptozotocin-induced diabetes, nor from caerulein-induced pancreatitis (38). In this study, Reg-II overexpression was moderate, and roughly at a similar level to the spontaneous Reg-II upregulation after caerulein treatment. It is possible that the advantage of transgenic overexpression of Reg-II over an endogenic Reg-II upregulation in response to caerulein is not significant, if the experimentally induced overexpression levels do not exceed the endogenic stress-induced upregulation. Reg-II is nevertheless markedly upregulated in acinar cells upon injury, and is hence evidently a stress response protein, albeit the precise function of Reg-II in pancreatic injury remains unclear. Apart from Reg-II upregulation, other reasons that may contribute to the exceptional disease resistance in the exocrine pancreas of keratin-deficient mice under basal conditions could include the unique intracellular localization of keratins in these cells and the shift between apicolateral and cytoplasmic filaments in response to cell injury, which is characteristic for the exocrine pancreas.
VII. Keratins in Human Pancreatitis and Pancreatic Cancer
Keratins, and other IFs among the cytoskeletal proteins are interesting to the medical field since they are associated with over 100 human diseases (45, 64, 69). Some keratin mutations are known to be directly causative of disease (e.g. several skin diseases), whilst mutations in simple epithelial keratins have been shown to predispose to various liver diseases. Moreover, since keratin expression, and post-translational modifications are frequently altered upon cell stress, keratins are also used as diagnostic biomarkers for diseases. One example of such histopathologies is the liver disease-associated formation of keratin aggregates, known as Mallory Denk bodies in hepatocytes (62, 69).
The prevalence of K8 mutations in human pancreatitis has been analyzed in a few studies, but no clear associations have been found. Two K8 variant mutations, Y54H and G62C, that predispose to cryptogenic liver disease (31) have been studied in this context. K8 G62C was first found to predispose to chronic pancreatitis (13). A later study found no significant correlation between the frequency of either K8 G62C or K8 Y54H and acute or chronic pancreatitis, or pancreatic cancer in a cohort of more than 2400 patients (75). Similarly, K8 G62C mutations were found not to be associated with familial, sporadic or alcoholic pancreatitis (55). However, a recent study has identified an association between the KRT8 gene and pancreatic cancer in a Japanese population genome-wide association study (40).
Changes in keratin expression levels during pancreatitis and pancreatic cancer have been analyzed in a few studies. K20 is a keratin that under normal circumstances has an expression pattern restricted to a few cell types and can therefore be used as a marker for certain cancers. As mentioned earlier, K20 expression has been reported in normal pancreatic duct cells in rats (7, 8), but the expression in humans is very low in the pancreas under basal conditions (78). However, K20 expression in human pancreatic duct cells is markedly induced in metastatic pancreatic cancer cells (78) and detection of K20 in pancreatic tumors or in blood or bone marrow samples from patients with pancreatic duct cell carcinomas, correlates with a worse prognosis (54, 60). Furthermore, K19, which in humans postnatally is restricted to duct cells, can be observed in pre-cancerous acinar-like cells before the appearance of metaplastic changes in cell morphology. Hence, K19 expression in neoplastic acinar cells is an early sign of metaplasia which precedes the fibrotic changes related to metaplasia (20).
VIII. Conclusions and Vision
During the last decades, keratin intermediate filaments have emerged as important cellular regulators and stress proteins of epithelial cells in many different organs. As the complexity of keratin-related functions has been unraveled, it has become evident that the function of keratins depends on the type of keratins expressed in the cells and their subcellular localization, as well as on the specific cell type and the cell-specific functions and regulatory pathways.
The keratin expression in the liver and the exocrine pancreas are similar, yet the susceptibilities to keratin deficiency-related injury are evidently different. The published studies relating to the role of keratins in pancreas and liver cells have provided some clues that help to explain the differences in stress tolerance.
The dramatic upregulation of the injury-response protein, Reg-II in the exocrine pancreas of K8 null and other keratin-deficient models, is probably one factor that protects K8-deficient mice from exocrine pancreatic injury (83). Indeed, this type of precautionary protection appears to take place also in skin cells, where keratin-mutant keratinocytes are similarly pre-prepared for injury through upregulation of basal level JUN-kinase activation and profuse activation of osmotic shock-induced stress pathways (15). Keratin deficiency may thus induce a chronic injury response that puts cells in an ‘alert state’, facilitating a swift cellular response to stress. The activation or inhibition of the same cell-signaling molecule may, however, have different consequences in different organs or under different circumstances, due to the complexity of the cell signaling pathways and downstream effects. It has, for instance, been shown that activation of transcription factor NF-κB has a protective, anti-apoptotic effect in liver injury and it has been suggested that the sensitivity of K8 null liver cells to apoptosis is linked to a defective activation of this transcription factor (37). Activation of NF-κB nevertheless appears to have a negative effect on pancreatic cell survival in caerulein-induced pancreatitis, since it enhances the inflammatory response (14). It is interesting to speculate whether the resistance of K8-deficient mice to caerulein-induced pancreatitis may be associated with interference with the NF-κB activation in pancreatitis (84), but a link between K8 deficiency and impeded NF-κB activation has not yet been reported for the exocrine pancreas.
Keratins in the exocrine pancreas may at first sight appear redundant, given that keratin deficiency is remarkably well tolerated under basal conditions as well as in some pancreatic injury models. The dynamic remodeling of keratins in response to exocrine pancreatic stress and the association of keratins with both Reg-II regulation as well as with inflammatory responses, such as NF-κB activation however contradicts the notion that keratins would lack a role in exocrine pancreatic injury responses. Rather than presenting an exceptional model organ in which keratins do not matter for stress tolerance, perhaps the exocrine pancreas really demonstrates the complexity of keratin-associated cell biology and the remarkable adaptability of the cells to counterbalance inherent weaknesses.
IX. Acknowledgements
The authors wish to acknowledge research funding during this time from The Novo Nordisk foundation, Finnish Diabetes foundation (DMT), The Academy of Finland (DMT), Sigrid Juselius foundation (DMT), The Medical Support foundation Liv och Hälsa (DMT), The Swedish Cultural foundation in Finland (CMA) and Jalmari and Rauha Ahokas Foundation (CMA).
X. References
- Abe M, Nata K, Akiyama T, Shervani NJ, Kobayashi S, Tomioka-Kumagai T, Ito S, Takasawa S, and Okamoto H. Identification of a novel Reg family gene, Reg IIIdelta, and mapping of all three types of Reg family gene in a 75 kilobase mouse genomic region. Gene 246: 111-122, 2000. PMID: 10767532.
- Alam CM, Silvander JS, Daniel EN, Tao GZ, Kvarnstrom SM, Alam P, Omary MB, Hanninen A, and Toivola DM. Keratin 8 modulates beta-cell stress responses and normoglycaemia. J Cell Sci 126: 5635-5644, 2013. PMID: 24144696.
- Alam CM, Silvander JSG, Helenius TO, and Toivola DM. Decreased levels of keratin 8 sensitize mice to streptozotocin-induced diabetes. Acta Physiol (Oxf) 224: e13085, 2018. PMID: 29719117.
- Asghar MN, Priyamvada S, Nystrom JH, Anbazhagan AN, Dudeja PK, and Toivola DM. Keratin 8 knockdown leads to loss of the chloride transporter DRA in the colon. Am J Physiol Gastrointest Liver Physiol 310: G1147-1154, 2016. PMID: 27125276.
- Baribault H, Penner J, Iozzo RV, and Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 8: 2964-2973, 1994. PMID: 7528156.
- Bouameur JE, Favre B, Fontao L, Lingasamy P, Begre N, and Borradori L. Interaction of plectin with keratins 5 and 14: dependence on several plectin domains and keratin quaternary structure. J Invest Dermatol 134: 2776-2783, 2014. PMID: 24940650.
- Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol 184: 234-239, 1998. PMID: 9614373.
- Bouwens L, Braet F, and Heimberg H. Identification of rat pancreatic duct cells by their expression of cytokeratins 7, 19, and 20 in vivo and after isolation and culture. J Histochem Cytochem 43: 245-253, 1995. PMID: 7532655.
- Brady GF, Kwan R, Bragazzi Cunha J, Elenbaas JS, and Omary MB. Lamins and Lamin-Associated Proteins in Gastrointestinal Health and Disease. Gastroenterology 154: 1602-1619 e1601, 2018. PMID: 29549040.
- Brembeck FH and Rustgi AK. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J Biol Chem 275: 28230-28239, 2000. PMID: 10859317.
- Carlotti F, Zaldumbide A, Ellenbroek JH, Spijker HS, Hoeben RC, and de Koning EJ. beta-Cell Generation: Can Rodent Studies Be Translated to Humans? J Transplant 2011: 892453, 2011. PMID: 22007286.
- Casanova ML, Bravo A, Ramirez A, Morreale de Escobar G, Were F, Merlino G, Vidal M, and Jorcano JL. Exocrine pancreatic disorders in transsgenic mice expressing human keratin 8. J Clin Invest 103: 1587-1595, 1999. PMID: 10359568.
- Cavestro GM, Frulloni L, Nouvenne A, Neri TM, Calore B, Ferri B, Bovo P, Okolicsanyi L, Di Mario F, and Cavallini G. Association of keratin 8 gene mutation with chronic pancreatitis. Dig Liver Dis 35: 416-420, 2003. PMID: 12868678.
- Chen X, Ji B, Han B, Ernst SA, Simeone D, and Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 122: 448-457, 2002. PMID: 11832459.
- D'Alessandro M, Russell D, Morley SM, Davies AM, and Lane EB. Keratin mutations of epidermolysis bullosa simplex alter the kinetics of stress response to osmotic shock. J Cell Sci 115: 4341-4351, 2002. PMID: 12376565.
- Deramaudt TB, Sachdeva MM, Wescott MP, Chen Y, Stoffers DA, and Rustgi AK. The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter. J Biol Chem 281: 38385-38395, 2006. PMID: 17056599.
- Elenbaas JS, Bragazzi Cunha J, Azuero-Dajud R, Nelson B, Oral EA, Williams JA, Stewart CL, and Omary MB. Lamin A/C Maintains Exocrine Pancreas Homeostasis by Regulating Stability of RB and Activity of E2F. Gastroenterology 154: 1625-1629 e1628, 2018. PMID: 29366840.
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, and Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119: 1763-1771, 2009. PMID: 19587451.
- Etienne-Manneville S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu Rev Cell Dev Biol 34: 1-28, 2018. PMID: 30059630.
- Grippo PJ and Sandgren EP. Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine pancreatic cancer progression in transgenic rodents. Int J Cancer 131: 1243-1248, 2012. PMID: 22024988.
- Gross DJ, Weiss L, Reibstein I, van den Brand J, Okamoto H, Clark A, and Slavin S. Amelioration of diabetes in nonobese diabetic mice with advanced disease by linomide-induced immunoregulation combined with Reg protein treatment. Endocrinology 139: 2369-2374, 1998. PMID: 9564847.
- Habtezion A, Toivola DM, Asghar MN, Kronmal GS, Brooks JD, Butcher EC, and Omary MB. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc Natl Acad Sci U S A 108: 1445-1450, 2011. PMID: 21220329.
- Habtezion A, Toivola DM, Butcher EC, and Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci 118: 1971-1980, 2005. PMID: 15840656.
- Haque WA, Vuitch F, and Garg A. Post-mortem findings in familial partial lipodystrophy, Dunnigan variety. Diabet Med 19: 1022-1025, 2002. PMID: 12647844.
- Helenius TO, Misiorek JO, Nystrom JH, Fortelius LE, Habtezion A, Liao J, Asghar MN, Zhang H, Azhar S, Omary MB, and Toivola DM. Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism. Mol Biol Cell 26: 2298-2310, 2015. PMID: 25904331.
- Herrmann H and Aebi U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb Perspect Biol 8, 2016. PMID: 27803112.
- Herrmann H, Strelkov SV, Burkhard P, and Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest 119: 1772-1783, 2009. PMID: 19587452.
- Hesse M, Zimek A, Weber K, and Magin TM. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur J Cell Biol 83: 19-26, 2004. PMID: 15085952.
- Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene 30: 127-138, 2011. PMID: 20890307.
- Kim S and Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol 11: 75-81, 2010. PMID: 20027187.
- Ku NO, Gish R, Wright TL, and Omary MB. Keratin 8 mutations in patients with cryptogenic liver disease. N Engl J Med 344: 1580-1587, 2001. PMID: 11372009.
- Ku NO, Michie S, Resurreccion EZ, Broome RL, and Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci U S A 99: 4373-4378, 2002. PMID: 11917136.
- Ku NO, Strnad P, Zhong BH, Tao GZ, and Omary MB. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology 46: 1639-1649, 2007. PMID: 17969036.
- Ku NO, Toivola DM, Strnad P, and Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12: 876-885, 2010. PMID: 20729838.
- Ku NO, Toivola DM, Zhou Q, Tao G, Zhong B, and Omary MB. Studying Simple Epithelial Keratins in Cells and Tissues. In: Intermediate Filament Cytoskeleton, edited by Omary MB, Coulombe, P.A. San Diego, CA, USA: Elsevier Academic Press, 2004, p. 489-517.
- Lahdeniemi IAK, Misiorek JO, Antila CJM, Landor SK, Stenvall CA, Fortelius LE, Bergstrom LK, Sahlgren C, and Toivola DM. Keratins regulate colonic epithelial cell differentiation through the Notch1 signalling pathway. Cell Death Differ 24: 984-996, 2017. PMID: 28475172.
- Lee J, Jang KH, Kim H, Lim Y, Kim S, Yoon HN, Chung IK, Roth J, and Ku NO. Predisposition to apoptosis in keratin 8-null liver is related to inactivation of NF-kappaB and SAPKs but not decreased c-Flip. Biol Open 2: 695-702, 2013. PMID: 23862017.
- Li B, Wang X, and Liu JL. Pancreatic acinar-specific overexpression of Reg2 gene offered no protection against either experimental diabetes or pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 299: G413-421, 2010. PMID: 20489047.
- Liao J and Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol 133: 345-357, 1996. PMID: 8609167.
- Lin Y, Nakatochi M, Hosono Y, Ito H, Kamatani Y, Inoko A, Sakamoto H, Kinoshita F, Kobayashi Y, Ishii H, Ozaka M, Sasaki T, Matsuyama M, Sasahira N, Morimoto M, Kobayashi S, Fukushima T, Ueno M, Ohkawa S, Egawa N, Kuruma S, Mori M, Nakao H, Adachi Y, Okuda M, Osaki T, Kamiya S, Wang C, Hara K, Shimizu Y, Miyamoto T, Hayashi Y, Ebi H, Kohmoto T, Imoto I, Kasugai Y, Murakami Y, Akiyama M, Ishigaki K, Matsuda K, Hirata M, Shimada K, Okusaka T, Kawaguchi T, Takahashi M, Watanabe Y, Kuriki K, Kadota A, Okada R, Mikami H, Takezaki T, Suzuki S, Yamaji T, Iwasaki M, Sawada N, Goto A, Kinoshita K, Fuse N, Katsuoka F, Shimizu A, Nishizuka SS, Tanno K, Suzuki K, Okada Y, Horikoshi M, Yamauchi T, Kadowaki T, Yu H, Zhong J, Amundadottir LT, Doki Y, Ishii H, Eguchi H, Bogumil D, Haiman CA, Le Marchand L, Mori M, Risch H, Setiawan VW, Tsugane S, Wakai K, Yoshida T, Matsuda F, Kubo M, Kikuchi S, and Matsuo K. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat Commun 11: 3175, 2020. PMID: 32581250.
- Loschke F, Seltmann K, Bouameur JE, and Magin TM. Regulation of keratin network organization. Curr Opin Cell Biol 32: 56-64, 2015. PMID: 25594948.
- Misiorek JO, Lahdeniemi IAK, Nystrom JH, Paramonov VM, Gullmets JA, Saarento H, Rivero-Muller A, Husoy T, Taimen P, and Toivola DM. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis 37: 777-786, 2016. PMID: 27234655.
- Neznanov NS and Oshima RG. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol 13: 1815-1823, 1993. PMID: 7680099.
- Odaka C, Loranger A, Takizawa K, Ouellet M, Tremblay MJ, Murata S, Inoko A, Inagaki M, and Marceau N. Keratin 8 is required for the maintenance of architectural structure in thymus epithelium. PLoS One 8: e75101, 2013. PMID: 24086449.
- Omary MB. "IF-pathies": a broad spectrum of intermediate filament-associated diseases. J Clin Invest 119: 1756-1762, 2009. PMID: 19587450.
- Omary MB, Ku NO, Strnad P, and Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest 119: 1794-1805, 2009. PMID: 19587454.
- Omary MB, Ku NO, Tao GZ, Toivola DM, and Liao J. "Heads and tails" of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci 31: 383-394, 2006. PMID: 16782342.
- Oriolo AS, Wald FA, Ramsauer VP, and Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp Cell Res 313: 2255-2264, 2007. PMID: 17425955.
- Ostrowski SE, Reilly AA, Collins DN, and Ramsingh AI. Progression or resolution of coxsackievirus B4-induced pancreatitis: a genomic analysis. J Virol 78: 8229-8237, 2004. PMID: 15254194.
- Pan X, Hobbs RP, and Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol 25: 47-56, 2013. PMID: 23270662.
- Pandol SJ. The Exocrine Pancreas. In: The Exocrine Pancreas. San Rafael (CA), 2010. PMID: 21634067.
- Park MK and Lee CH. Effects of cerulein on keratin 8 phosphorylation and perinuclear reorganization in pancreatic cancer cells: Involvement of downregulation of protein phosphatase 2A and alpha4. Environ Toxicol 31: 2090-2098, 2016. PMID: 26303380.
- Roux A, Gilbert S, Loranger A, and Marceau N. Impact of keratin intermediate filaments on insulin-mediated glucose metabolism regulation in the liver and disease association. FASEB J 30: 491-502, 2016. PMID: 26467793.
- Schmitz-Winnenthal FH, Volk C, Helmke B, Berger S, Hinz U, Koch M, Weitz J, Kleeff J, Friess H, Zoller M, Buchler MW, and Z'Graggen K. Expression of cytokeratin-20 in pancreatic cancer: an indicator of poor outcome after R0 resection. Surgery 139: 104-108, 2006. PMID: 16364723.
- Schneider A, Lamb J, Barmada MM, Cuneo A, Money ME, and Whitcomb DC. Keratin 8 mutations are not associated with familial, sporadic and alcoholic pancreatitis in a population from the United States. Pancreatology 6: 103-108, 2006. PMID: 16327287.
- Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, and Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol 174: 169-174, 2006. PMID: 16831889.
- Silvander JSG, Kvarnstrom SM, Kumari-Ilieva A, Shrestha A, Alam CM, and Toivola DM. Keratins regulate beta-cell mitochondrial morphology, motility, and homeostasis. FASEB J 31: 4578-4587, 2017. PMID: 28666985.
- Snider NT and Omary MB. Assays for Posttranslational Modifications of Intermediate Filament Proteins. Methods Enzymol 568: 113-138, 2016. PMID: 26795469.
- Snider NT and Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15: 163-177, 2014. PMID: 24556839.
- Soeth E, Grigoleit U, Moellmann B, Roder C, Schniewind B, Kremer B, Kalthoff H, and Vogel I. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol 131: 669-676, 2005. PMID: 16136352.
- Spazierer D, Raberger J, Gross K, Fuchs P, and Wiche G. Stress-induced recruitment of epiplakin to keratin networks increases their resistance to hyperphosphorylation-induced disruption. J Cell Sci 121: 825-833, 2008. PMID: 18285451.
- Strnad P, Paschke S, Jang KH, and Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol 28: 209-216, 2012. PMID: 22450891.
- Strnad P, Stumptner C, Zatloukal K, and Denk H. Intermediate filament cytoskeleton of the liver in health and disease. Histochemistry and Cell Biology 129: 735-749, 2008. PMID: 18443813.
- Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, Sim SY, Goh WL, Ng KW, Simpson JA, Chee LL, Eng GH, Li B, Lunny DP, Chuon D, Venkatesh A, Khoo KH, McLean WH, Lim YP, and Lane EB. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat 29: 351-360, 2008. PMID: 18033728.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50: 537-546, 2001. PMID: 11829314.
- Terazono K, Yamamoto H, Takasawa S, Shiga K, Yonemura Y, Tochino Y, and Okamoto H. A novel gene activated in regenerating islets. J Biol Chem 263: 2111-2114, 1988. PMID: 2963000.
- Toivola D, Ostrowski S, Baribault H, Magin T, Ramsingh A, and Omary M. Keratins provide virus-dependent protection or predisposition to injury in coxsackievirus-induced pancreatitis. Cell Health and Cytoskeleton 1: 51-65, 2009.
- Toivola DM, Baribault H, Magin T, Michie SA, and Omary MB. Simple epithelial keratins are dispensable for cytoprotection in two pancreatitis models. Am J Physiol Gastrointest Liver Physiol 279: G1343-1354, 2000. PMID: 11093958.
- Toivola DM, Boor P, Alam C, and Strnad P. Keratins in health and disease. Curr Opin Cell Biol 32: 73-81, 2015. PMID: 25599598.
- Toivola DM, Ku NO, Ghori N, Lowe AW, Michie SA, and Omary MB. Effects of keratin filament disruption on exocrine pancreas-stimulated secretion and susceptibility to injury. Exp Cell Res 255: 156-170, 2000. PMID: 10694432.
- Toivola DM, Nakamichi I, Strnad P, Michie SA, Ghori N, Harada M, Zeh K, Oshima RG, Baribault H, and Omary MB. Keratin overexpression levels correlate with the extent of spontaneous pancreatic injury. Am J Pathol 172: 882-892, 2008. PMID: 18349119.
- Toivola DM, Nieminen MI, Hesse M, He T, Baribault H, Magin TM, Omary MB, and Eriksson JE. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 34: 1174-1183, 2001. PMID: 11732007.
- Toivola DM, Omary MB, Ku NO, Peltola O, Baribault H, and Eriksson JE. Protein phosphatase inhibition in normal and keratin 8/18 assembly-incompetent mouse strains supports a functional role of keratin intermediate filaments in preserving hepatocyte integrity. Hepatology 28: 116-128, 1998. PMID: 9657104.
- Toivola DM, Tao GZ, Habtezion A, Liao J, and Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 15: 608-617, 2005. PMID: 16202602.
- Treiber M, Schulz HU, Landt O, Drenth JP, Castellani C, Real FX, Akar N, Ammann RW, Bargetzi M, Bhatia E, Demaine AG, Battagia C, Kingsnorth A, O'Reilly D, Truninger K, Koudova M, Spicak J, Cerny M, Menzel HJ, Moral P, Pignatti PF, Romanelli MG, Rickards O, De Stefano GF, Zarnescu NO, Choudhuri G, Sikora SS, Jansen JB, Weiss FU, Pietschmann M, Teich N, Gress TM, Ockenga J, Schmidt H, Kage A, Halangk J, Rosendahl J, Groneberg DA, Nickel R, and Witt H. Keratin 8 sequence variants in patients with pancreatitis and pancreatic cancer. J Mol Med (Berl) 84: 1015-1022, 2006. PMID: 17039343.
- Uitto J, Richard G, and McGrath JA. Diseases of epidermal keratins and their linker proteins. Exp Cell Res 313: 1995-2009, 2007. PMID: 17531221.
- Waseem A, Gough AC, Spurr NK, and Lane EB. Localization of the gene for human simple epithelial keratin 18 to chromosome 12 using polymerase chain reaction. Genomics 7: 188-194, 1990. PMID: 1693358.
- Wildi S, Kleeff J, Maruyama H, Maurer CA, Friess H, Buchler MW, Lander AD, and Korc M. Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res 5: 2840-2847, 1999. PMID: 10537351.
- Windoffer R, Beil M, Magin TM, and Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. Journal of Cell Biology 194: 669-678, 2011. PMID: 21893596.
- Wogenstein KL, Szabo S, Lunova M, Wiche G, Haybaeck J, Strnad P, Boor P, Wagner M, and Fuchs P. Epiplakin deficiency aggravates murine caerulein-induced acute pancreatitis and favors the formation of acinar keratin granules. PLoS One 9: e108323, 2014. PMID: 25232867.
- Yi H, Yoon HN, Kim S, and Ku NO. The role of keratins in the digestive system: lessons from transgenic mouse models. Histochem Cell Biol 150: 351-359, 2018. PMID: 30039330.
- Zhang JS, Wang L, Huang H, Nelson M, and Smith DI. Keratin 23 (K23), a novel acidic keratin, is highly induced by histone deacetylase inhibitors during differentiation of pancreatic cancer cells. Genes Chromosomes Cancer 30: 123-135, 2001. PMID: 11135429.
- Zhong B, Strnad P, Toivola DM, Tao GZ, Ji X, Greenberg HB, and Omary MB. Reg-II is an exocrine pancreas injury-response product that is up-regulated by keratin absence or mutation. Mol Biol Cell 18: 4969-4978, 2007. PMID: 17898082.
- Zhong B, Zhou Q, Toivola DM, Tao GZ, Resurreccion EZ, and Omary MB. Organ-specific stress induces mouse pancreatic keratin overexpression in association with NF-kappaB activation. J Cell Sci 117: 1709-1719, 2004. PMID: 15075232.
- Zhou Q, Toivola DM, Feng N, Greenberg HB, Franke WW, and Omary MB. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell 14: 2959-2971, 2003. PMID: 12857878.