Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2016.1
| Attachment | Size |
|---|---|
| 581.18 KB |
Abstract:
1. Pathophysiological Considerations
Alterations of the pancreatic microperfusion are an early event in the course of pancreatitis irrespective of the underlying etiology (10). They result in reduced blood flow, capillary leakage, pancreatic and peripancreatic edema and transmigration of inflammatory cells. The source of proinflammatory mediators leading to these events have not been fully identified, but studies indicate that acinar and stellate cells as well as resident immune cells can all respond to pancreatic injury by secreting proinflammatory cytokines such as IL-1b, IL-6 and TNF-α (23). Ultimately the decreased microperfusion and activation of the endothelium will also lead to hypercoagulability which, in turn, aggravates pancreatic hypoperfusion and hypoxia. The lack of oxygen as well as reperfusion damage will lead to pancreatic necrosis with sometimes catastrophic consequences such as infected pancreatic collections, sepsis, bleeding and death (9). Systemically multiorgan failure due to systemic inflammatory response syndrome, hypoperfusion and shock are common events in severe forms of acute pancreatitis, leading to mortality rates close to 50% in some patient cohorts (2).
Early fluid resuscitation could thus help to restore local pancreatic perfusion, counteract systemic hypotension and thus prevent secondary organ failure due to fluid sequestration. The critical question in this context is how much fluid replacement will be optimal to improve outcome and how much will lead to fluid overload with negative consequences such as abdominal compartment syndrome (7, 25).
2. Estimation of Fluid Requirement
Early and adequate fluid resuscitation remains the corner stone of initial treatment in acute pancreatitis and probably has the most detrimental consequences if not properly administered. An observational study including 403 patients from two prospectively collected cohorts showed an association between early fluid deficit and the development of pancreatic fluid collections, pancreatic necrosis, persistent organ failure and the length of hospital stay (13). In a smaller cohort Scottish patients who died from acute pancreatitis received significantly less fluids within 48 h after admission than survivors (16). In a small pooled analysis of 44 patients with and without necrotizing pancreatitis it was shown that, although similar amounts of fluid were administered in both groups, only those with a high hematocrit after 24 h developed necrosis during their subsequent course of pancreatitis (3).
Although the need for fluid resuscitation in these patients is widely accepted, the prediction of the extent of fluid sequestration and thus the clinical outcome remains a challenge. Clinically used scoring systems as well as recently published studies focus on surrogates for fluid sequestration as predictors of outcome and indicators for goal directed administration of fluids in the early phase. These include hematocrit, blood urea nitrogen (BUN), creatinine, heart rate, mean arterial pressure (MAP) and central venous pressure (CVP) (16, 18, 19, 24, 28, 30).
3. Choice of Fluids
Based on the current evidence from pancreatitis specific, as well as general critical care studies, balanced crystalloid solutions such as Ringers´ Lactate should be used for fluid resuscitation in acute pancreatitis patients. In a randomized controlled trail including 40 North American patients Wu et al. showed that patients randomly assigned to receive Lactated Ringers´ had a significant reduction in systemic inflammatory response syndrome (SIRS; 84% reduction vs. 0%; χ²p=0,035) and CRP levels (mean 51 mg/L vs. 104 mg/L; ANOVA p=0,018) compared to patients receiving normal saline (29). One advantage of balanced solutions is their favorable effect on acid base metabolism. Experimental animal studies suggest that lactate has a direct anti-inflammatory effect via the GPR81 receptor and the cellular inflammasome (8,11). Studies outside the pancreatitis field could also show that hyperchloremic acidosis induced by infusion of large amounts of saline can lead to a worse outcome with an increased risk for kidney injury, thus leaving normal saline to be only a second choice of fluids in critically ill patients (12). In pancreatitis patients resuscitation with Ringers´ Lactate led to a significantly reduced rate of acidosis with a reverse correlation of bicarbonate to CRP levels (29).
Hydroxyethyl starch (HES) is a colloid fluid that has been widely used for plasma expansion in critically ill patients. A large randomized, blinded intensive care patient trial not specific for pancreatitis, analyzed the outcome of 798 ICU patients receiving either HES or crystalloids. The study found that the use of HES was associated with a higher mortality as compared to Ringers´ acetate (201/398; 51% vs. 172/400; 43%; p=0,03) and increased the risk for renal failure and the need for renal replacement therapy (87/398; 22% vs. 65/400; 16%; p=0.04) (20). The unfavorable effect of HES did not reach significance in the longterm mortality after 6 month or one year, which may be due to insufficient power of the study for this endpoint. However, HES failed to show any longterm superiority (21). A previous study by Brunkhorst and col-leagues showed similar results (4). For pancreatitis patients Mole et al. observed an increased use of HES in a group of patients that had died from acute pancreatitis (16). There is one small study in acute pancreatitis showing that a combination of Ringers´ lactate and HES reduced the mean intraabdominal pressure and the need for mechanical ventilation within the first week of acute pancreatitis when compared to Ringers´ lactate alone (5). However, due to the small sample sizes the grade of evidence remains too low to currently recommend the use of HES in acute pancreatitis in the light of the larger ICU studies. In summary, balanced, full electrolyte crystalloid solutions are currently recommended for initial fluid resuscitation in acute pancreatitis, with the limitation that only Ringers´ lactate has been investigated for this purpose so far. In patients with hypercalcemia, calcium-free normal saline serves as an alternative.
4. Course of Fluid Resuscitation
Earlier observational studies on pancreatitis patients concluded that early and aggressive fluid therapy improved outcome and prevented necrosis (6, 16, 26). The first randomized controlled trial to investigate this question originated from China and, somewhat unexpectedly, showed that overly aggressive fluid administration can be harmful when compared to controlled fluid expansion. The rapid fluid expansion group received crystalloids or colloids at a rate 10-15 ml/kg b.w. per hour and was at higher risk for mechanical ventilation (94.4% vs. 65%) and death (30.6% vs 10%) when compared to controlled fluid expansion group with 5-10 ml/kg of body weight per hour. The amounts of fluid given only differed over the first 24 h, but were similar over the subsequent 4 days. The same applied to hematocrit levels, which were also transiently lower in the aggressive fluid treatment group during the first day (15). In a subsequent, larger, randomized trial from the same institution patients were assigned to meet a resuscitation goal of above or below a 35% hematocrit within 48 h. Again, patients with more aggressive treatment receiving more fluids in the early course had a worse outcome with higher APACHE II scores, higher risk for sepsis and death (14). Taken together these results suggest that fluids should be given at moderate rates of 5-10 ml/kg of body weight over the first 24 h aiming for a total volume of 2500 ml to 4000 ml. Recently the concept of goal directed fluid resuscitation has been more heavily investigated both in and outside the pancreatitis field. Parameters that have been investigated are BUN, hematocrit, CVP, blood pressure, heart rate and urine output.
Wu et al. concluded from a small randomized trial, that BUN, despite its prognostic values, does not help to guide fluid resuscitation because the total amount of fluid administered as well as the prevalence of SIRS and CRP values were similar between the group receiving BUN-guided fluids and the control arm (29). An observational study concluded that central venous pressure might be a misleading parameter to guide fluid administration because patients with high CVP were more likely to receive vasopressors and were at a higher risk for death (16). The most controversial parameter lately was hematocrit. A retrospective study by Brown and colleagues showed that all 12 patients with persistently high hematocrit of above 44% after 24 h died, whereas Mao et al. convincingly showed that rapid hemodilution to a hematocrit of below 35% within 48 h also puts the patient at higher risk for pancreatitis related death (14). Pathophysiologically these effects could be explained by kidney damage and tissue hypoperfusion in cases of high hematocrit, and impaired oxygen delivery, coagulation failure and decreased migration of inflammatory cells in the presence of a low hematocrit.
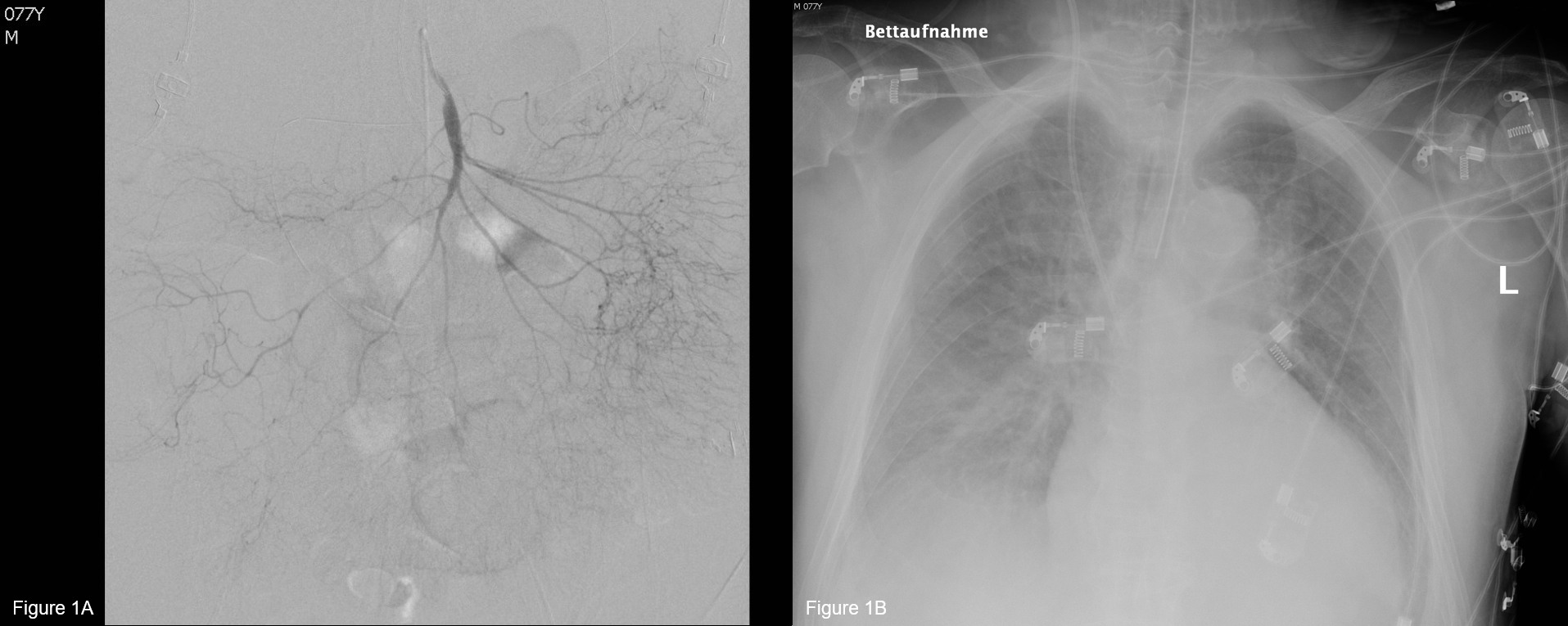
Figure 1. Complications of fluid overload in severe acute pancreatitis.A 77 years old male patient with biliary pancreatitis and preexisting congestive heart failure due to long lasting arterial hypertension and aortic valve stenosis was resuscitated with a total of 2500 ml balanced crystalloid infusion over the first 24 h. Within 48 h from admission he developed respiratory failure with increasing oxygen requirements and was consequently admitted to the intensive care unit. He developed severe ARDS due to fluid overload and cardiac decompensation, was intubated and ventilated (Figure 1B). Later in his course he also developed increased intraabdominal pressure and abdominal compartment syndrome with central venous congestion followed by non-occlusive mesenteric ischemia. Figure 1A shows the angiogram of the superior mesenteric artery with narrowing of all vessels and distal hypoperfusion. A papaverin catheter was inserted, but despite maximal escalation of treatment the patient died of multiorgan failure and sepsis.
In general the physician in charge needs to consider coexisting conditions such congestive heart failure or pulmonary disease which limit the tolerance towards fluid administration drastically and thus adjust fluid management. The study by Mao further suggests that a heart rate of <120 bpm, a mean arterial pressure of 65-85 mmHg and urine output of 0.5-1 ml/kg/h can be used to non-invasively estimate fluid requirements. However, low urine output can also be a consequence of acute tubular necrosis in which case more fluid administration will lead to fluid overload and respiratory failure. Three large multicenter randomized trials conducted in Australia/New Zealand, the United States and the United Kingdom uniformly concluded that early goal directed fluid therapy was not superior to usual care protocols (1, 17, 22). This supports the conclusion that predictive factors that can guide the fluid treatment in acute pancreatitis patients remain to be identified. Whether modern hemodynamic monitoring using e.g. thermodilution methods can be of benefit for a sub-set of patients is currently under investigation (31).
5. IAP/APA Guideline Recommendations
During the APA annual meeting 2012 an expert panel developed new evidence-based guidelines for the management of acute pancreatitis (27). For fluid resuscitation the following was recommended.
- Ringer´s lactate is recommended for initial fluid resuscitation in acute pancreatitis. (GRADE 1B, strong agreement)
- Goal directed intravenous fluid therapy with 5-10 ml/kg/h should be used initially until resuscitation goals are reached. (GRADE 1B, weak agreement)
- The preferred approach to assessing the response to fluid resuscitation should be based on one or more of the following: (1) non-invasive clinical targets of heart rate <120/min, mean arterial pressure between 65 and 85 mmHg (8.7 - 11.3 kPa), and urinary output >0.5 - 1 ml/kg/h, (2) invasive clinical targets of stroke volume variation, and intrathoracic blood volume determination, and (3) biochemical targets of hematocrit 35 - 44%. (GRADE 2B, weak agreement)
6. References
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371: 1496–1506, 2014. PMID: 25272316.
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 62(1):102-11, 2013. PMID: 23100216.
- Brown A, Baillargeon J-D, Hughes MD, Banks PA. Can fluid resuscita-tion prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2: 104–107, 2002. PMID: 12123089.
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358: 125–139, 2008. PMID: 18184958.
- Du X-J, Hu W-M, Xia Q, Huang Z-W, Chen G-Y, Jin X-D, et al. Hydroxyethyl starch resuscitation reduc-es the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas 40: 1220–1225, 2011. PMID: 21775917.
- Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, et al. Faster rate of initial fluid resuscita-tion in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology 9: 770–776, 2009. PMID: 20110744.
- Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, Zygun DA, et al. Risk factors for intraabdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and metaanalysis. Crit Care 17: R249, 2013. PMID: 24144138.
- Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146(7):1763-74, 2014. PMID: 24657625.
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 386: 85–96, 2015. PMID: 25616312.
- Lerch MM, Weidenbach H, Gress TM, Adler G. Effect of kinin inhibition in experimental acute pancreatitis. Am J Physiol 269:G490-9, 1995. PMID: 7485500.
- Lerch MM, Conwell DL, Mayerle J. The anti-inflammasome effect of lactate and the lactate GPR81-receptor in pancreatic and liver inflammation. Gastroenterology 146(7):1602-5, 2014. PMID: 24780214.
- Lobo DN, Awad S. Should chloride-rich crystalloids remain the main-stay of fluid resuscitation to prevent “pre-renal” acute kidney injury? Kidney Int 86: 1096–1105, 2014. PMID: 24717302.
- de-Madaria E, Banks PA, Moya-Hoyo N, Wu BU, Rey-Riveiro M, Acevedo-Piedra NG, et al. Early factors associated with fluid sequestration and outcomes of pa-tients with acute pancreatitis. Clin Gastroenterol Hepatol 12: 997–1002, 2014. PMID: 24183957.
- Mao E-Q, Fei J, Peng Y-B, Huang J, Tang Y-Q, Zhang S-D. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 123: 1639–1644, 2010. PMID: 20819621.
- Mao E, Tang Y, Fei J, Qin S, Wu J, Li L, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 122: 169–173, 2009. PMID: 19187641.
- Mole DJ, Hall A, McKeown D, Garden OJ, Parks RW. Detailed fluid resuscitation profiles in patients with severe acute pancreatitis. HPB 13: 51–58, 2011. PMID: 21159104.
- Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372: 1301–1311, 2015. PMID: 25776532.
- Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 142: 1476–1482; quiz e15–16, 2012. PMID: 22425589.
- Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol 104: 164–170, 2009. PMID: 19098865.
- Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 367: 124–134, 2012. PMID: 22738085.
- Perner A, Haase N, Winkel P, Guttormsen AB, Tenhunen J, Klemenzson G, et al. Long-term out-comes in patients with severe sepsis randomised to resuscitation with hydroxyethyl starch 130/0.42 or Ringer’s acetate. Intensive Care Med 40: 927–934, 2014. PMID: 24807084.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 370: 1683–1693, 2014. PMID: 24635773.
- Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, Scharffetter-Kochanek K, et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 62(3):430-9, 2013. PMID: 26228362.
- Trikudanathan G, Navaneethan U, Vege SS. Current controversies in fluid resuscitation in acute pancreatitis: a systematic review. Pancreas 41: 827–834, 2012. PMID: 22781906.
- Trikudanathan G, Vege SS. Current concepts of the role of abdominal compartment syndrome in acute pancreatitis - an opportunity or mere-ly an epiphenomenon. Pancreatology 14: 238–243, 2014. PMID: 25062870.
- Wall I, Badalov N, Baradarian R, Iswara K, Li JJ, Tenner S. Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas 40: 547–550, 2011. PMID: 21499208.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 13: e1–15, 2013. PMID: 24054878.
- Wu BU, Bakker OJ, Papachristou GI, Besselink MG, Repas K, van Santvoort HC, et al. Blood urea nitrogen in the early assessment of acute pan-creatitis: an international validation study. Arch Intern Med 171: 669–676, 2011. PMID: 21482842.
- Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 9: 710–717.e1, 2011. PMID: 21645639.
- Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 137: 129–135, 2009. PMID: 19344722.
- Early Goal-directed Volume Resuscitation in Severe Acute Pancreatitis - Full Text View - ClinicalTrials.gov [Online]. https://clinicaltrials.gov/ct2/show/NCT00894907 [25 Oct. 2015].


