Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2018.19
| Attachment | Size |
|---|---|
| 1.37 MB |
Abstract
Background/Objectives: The aim of this review is to provide a valuable and focused source of information for understanding and evaluating pancreatic manifestations of patients with celiac disease (CD). Methods: The review encompasses the current diagnostic criteria of CD and disease related prevalence and mechanisms of secondary exocrine pancreatic insufficiency (EPI) and the risk and mechanisms of pancreatitis. Topics also include the prevalence of CD in idiopathic pancreatitis, when to investigate and treat other causes of steatorrhea, particularly EPI with pancreatic enzyme replacement therapy (PERT). Conclusions: In “classical” CD the prevalence of EPI ranges 0-77.8%, on the basis of secretagogue and test meal evoked direct pancreatic function tests. Severe EPI (sufficient to cause steatorrhea) is uncommon, ranging in prevalence from 0-18%, and is usually reversible. Major mechanisms of EPI in CD include disruption of enteric mediated hormone secretion, luminal dilution of digestive enzymes and bile acids, malnutrition and immune mediated inflammation. CD associates with an increased risk of developing acute pancreatitis and chronic pancreatitis. Conversely, there is likely an unrecognized incidence of CD in idiopathic pancreatitis. In patients with CD and persistent malabsorption or weight loss despite a gluten free diet (GFD), EPI should be one of several considerations and a trial of PERT offered to patients.
1. Introduction
Celiac disease (CD) is an autoimmune condition induced by gluten exposure in genetically predisposed individuals. The estimated prevalence is approximately 1% (20,23,53,60). CD manifestations include gastrointestinal (GI), extra-intestinal with or without GI disease, or asymptomatic disease. To improve consensus with CD terminology, the 2011 Oslo multidisciplinary task force (29) defined and recommended use of 9 terms. The two major CD terms are “classical” (signs and symptoms of malabsorption) and “non-classical” (symptoms present but malabsorption signs/symptoms are absent) disease. Seven additional CD terms include subclinical, symptomatic, refractory, potential, autoimmunity, genetically at risk and non-celiac gluten sensitivity. This report and other reviews (23, 53) lack a discussion of pancreatic disease associations. The aim of this review is to address this gap in clinical information by reviewing secondary exocrine pancreatic insufficiency (EPI) and pancreatitis associated with CD.
2. Exocrine pancreatic insufficiency (EPI) in Celiac Disease
In “classical” CD, numerous studies have reported an association with EPI, diagnosed by measurements of pancreatic enzyme or bicarbonate secretions (Tables 1 and 2) evoked by the intravenous (i.v.) administration of GI hormone analogues for secretin (5,17,43), Cholecystokinin (CCK) (8,14,39,51,72), or cholinergic agonists (19), ingestion of test meals (22,64,70), and duodenal infusion of amino acids (14). In case series having at least 5 patients the overall prevalence of EPI in "classical" CD ranges 0-77.8% (Table 1) but varies considerably by the specific pancreatic function test (PFT): 0-15.4% with i.v. secretin, 22.7-77.8% with i.v. CCK analogues, and 42.9-58% with test meal. Severe EPI (<10% normal enzyme outputs evoked by CCK (51)) sufficient to contribute to steatorrhea is uncommon, ranging in prevalence from 0-18.8% in series with at least 5 patients with active CD (table 2) (5,8,43,51,72), and described in only 10 cases confirmed by direct PFTs or clinical history and autopsy (5,8,18,19,43,51,72). Of these ten cases, 6 responded to pancreatin (5,19,51,72), 1 responded to GFD alone (18), and 3 were resistant to both therapies (43).
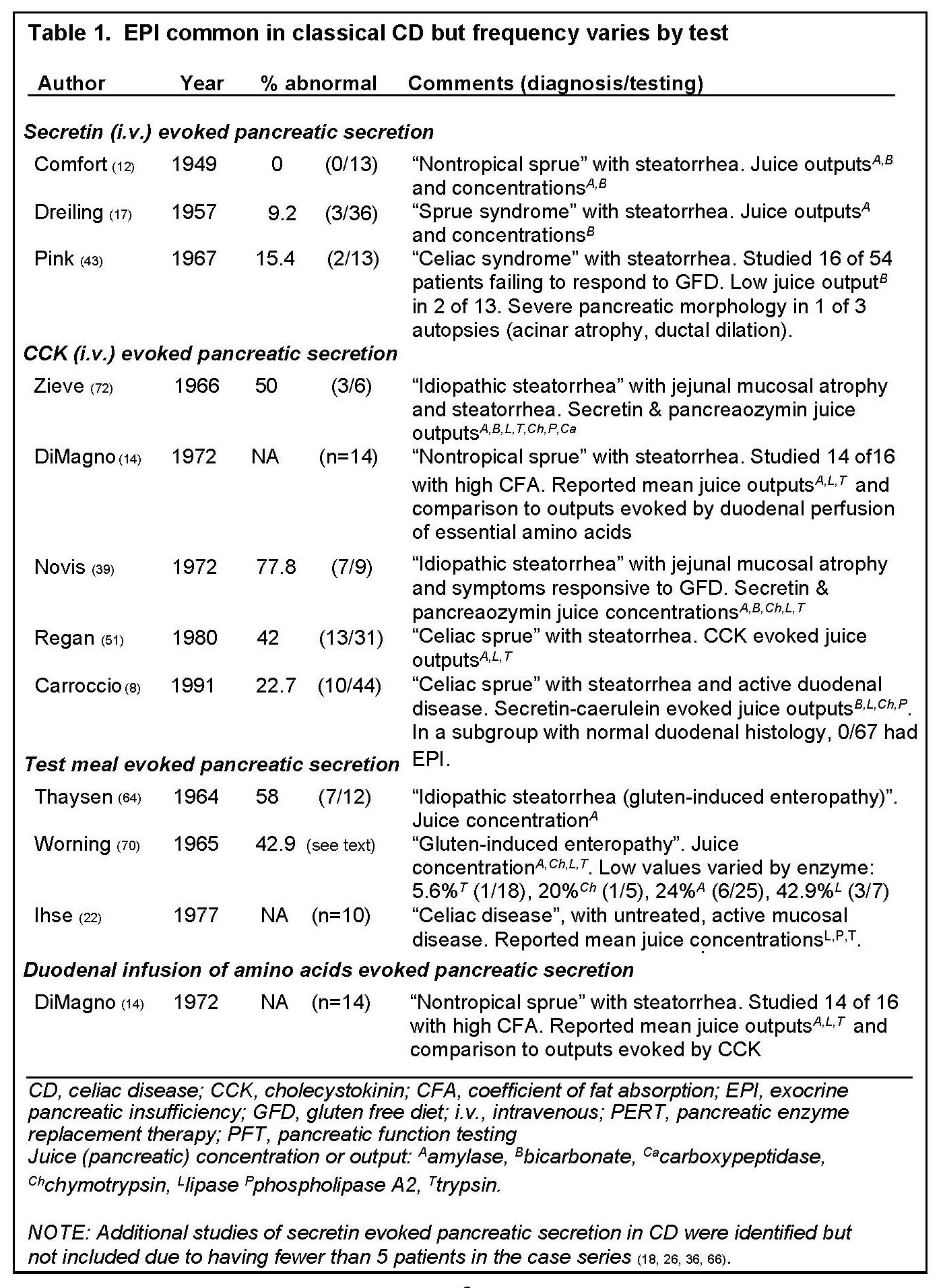
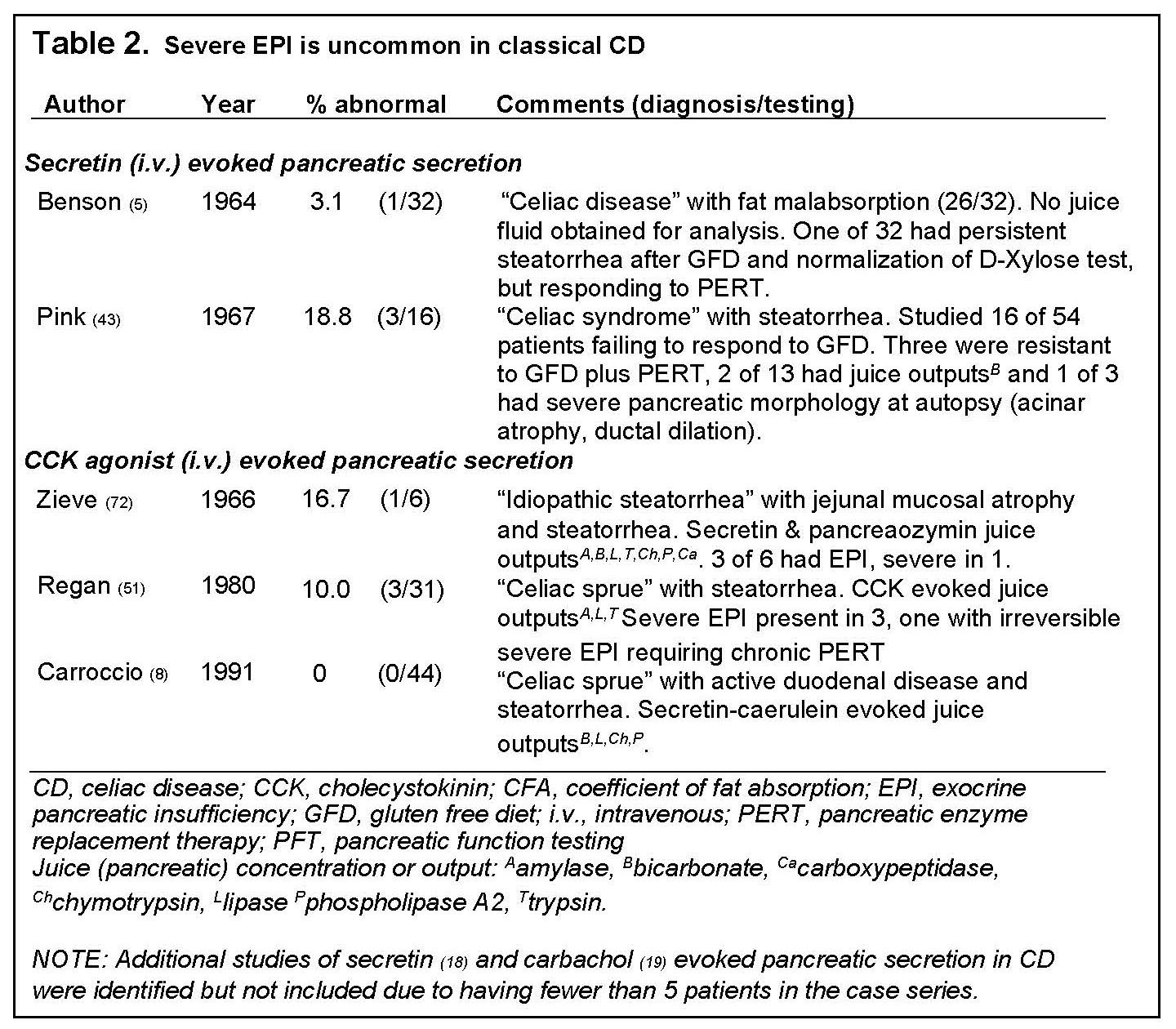
Intestinal damage, a histological feature of "classical" CD, correlates with the degree of decline in exocrine pancreatic function (38). Following initiation of gluten free diet (GFD), EPI is typically reversible (18,66); some patients slowly resolve EPI and malapsorption. Only 7 cases with clinical follow-up have been reported to have irreversible EPI, including 5 confirmed by direct PFT (5,19,43,51), one by clinical characteristics and autopsy (43), and one by low serum immunoreactive trypsin with steatorrhea improving with PERT (67).
3. Mechanisms of EPI in Celiac Disease
Four major mechanisms of EPI in CD have been proposed, including, disruption of enteric mediated hormone secretion, luminal dilution of bile acids and pancreatic enzymes, malnutrition and immune mediated inflammation (discussed under Pancreatitis).
Disruption of enteric mediated hormone secretion
Two methodologic approaches have been used to establish the disruption in enteric-mediated hormone secretion in untreated CD (Figure 1), causing reduced pancreatic secretion of digestive juice and gallbladder emptying: pancreatic function testing (in response to amino acids, diet and exogenous hormones) and measurement of enteric hormones secreted in blood.
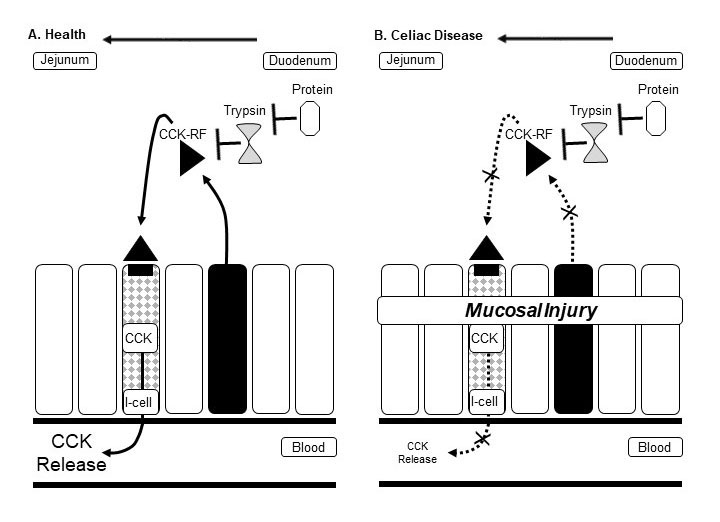
Figure 1. Schematic of enterocyte damage impairing cholecystokinin (CCK) signaling. In health (A) the small intestine enterocytes (solid black) release into the lumen CCK releasing factor (CCK-RF), which has paracrine effects on I-cells, leading to CCK release into the circulation, which in turn acts via vagal cholinergic pathways to stimulate pancreatic secretion (41). Signaling is sensitive to luminal trypsin (from endogenous pancreatic juice or exogenously administered pancreatic enzymes), which degrades intraluminal CCK-RF. In active celiac disease (B) associated with enterocyte injury, CCK signaling is impaired (14) at the level of enterocyte release of CCK-RF into the small bowel lumen and enterocyte (I-cell) release of CCK into the circulation.
In untreated CD, reduced release of enteric hormones has been postulated to cause secondary EPI (14,22,70). In these studies, EPI was diagnosed by direct pancreatic function testing, with measurement of enzyme and bicarbonate outputs or concentrations in duodenal juice following test meals (14,22,70) and duodenal infusion of amino acids (14). For example, the 1973 study by DiMagno and colleagues (Figure 2) showed that patients with untreated CD had evidence of reduced pancreatic enzyme outputs and gallbladder emptying in response to intraluminal amino acid infusion, which evokes endogenous cholecystokinin (CCK) secretion, but that exocrine pancreatic- and gallbladder functions normalized in response to exogenous intravenous infusion of CCK-pancreozymin (14).
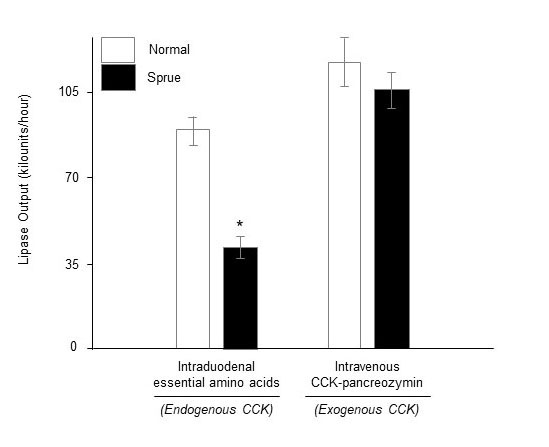
Figure 2. Impaired cholecystokinin-pancreozymin (CCK-PZ) secretion in celiac disease. Mean (± SE) pancreatic enzyme outputs per hour in health (n=12) and celiac disease with steatorrhea (n=14) in response to intravenous CCK-PZ stimulation (0.25 Crick-Harper-Raper units/kg/min), representing exogenous CCK, and intraduodenal essential amino acids (EAA, 78 mM), the latter stimulating endogenous CCK release. *P<0.01 vs. intraduodenal EAA treated normal. Adapted from Gastroenterology (14).
In addition, these investigators fed two test meals four hours apart. As we summarized previously, “Postprandially these abnormalities resulted in maldigestion of fat due to asynchronization between transit of the meal and delayed and reduced secretion of pancreatic enzymes and bile into the small intestine that occurred during the first 30 minutes after eating. After the initial 30 postprandial minutes, dilution of intraluminal content secondary to abnormalities of fluid and electrolyte absorption/secretion contributed to impaired fat digestion. Fat maldigestion was worse after a second meal” (15). Restoration of pancreatic function is possible by introduction of a GFD with resultant enteric healing. Conversely, EPI is generally a manifestation of persistently active enteric disease (8,43,67), and as discussed above is uncommonly severe and usually reversible.
Small intestinal enterocytes synthesize, store and secrete the enteric hormones CCK and secretin (25). In untreated CD, associated with intestinal mucosal atrophy, blood levels of these hormones are reduced. Compared to controls, patients with untreated CD have lower blood CCK levels while fasting (6) and postprandially (6,32) and have lower postprandial gallbladder emptying (32). Treatment with GFD normalized both postprandial CCK and gallbladder emptying (32). Similarly, reduced blood secretin levels are also present in untreated CD, measured following duodenal perfusion of hydrochloric acid (24,40,52,69), but normalizes after GFD treatment (24). Data is conflicting whether secretin levels are low in the unstimulated/fasting state (24,40,52).
Luminal dilution of bile acids and pancreatic enzymes
As discussed above, CD disease associates with decreased luminal concentrations of pancreatic enzymes and bile acids due to disruption of enteric mediated hormone pancreatic secretion and gallbladder emptying. Decreased luminal concentrations of pancreatic enzymes and bile acids may be further diminished due to a luminal dilution from reduced absorption of fluid and electrolytes (21,59). The latter has been attributed to small bowel injury with impaired absorption of nutrients resulting in an increased prandial luminal osmotic load (59), but also to reduced mucosal pore size in active CD (21), both of which are reversible.
Malnutrition
EPI, diagnosed by diminished pancreatic enzyme secretions evoked by administration of intravenous secretagogues, associates with a spectrum of protein-calorie malnutrition (PCM) disorders, including kwashiorkor (with features of muscle wasting, peripheral edema, skin lesions, growth retardation, hypoalbuminemia and deficiencies of essential amino acids) and marasmus (features of kwashiorkor but without edema or skin lesions) (3,44,57,63). Because severe cases of “classical” CD can give rise to malnutrition, it has been postulated that EPI in CD may also be due in part to malnutrition (66,70).
There are two postulated mechanisms for EPI with PCM but these have doubtful relevance to CD. First, deficiencies of essential amino acids are common in PCM (3) and would be expected to impair protein synthesis of digestive enzymes. Evidence to support this mechanism is that dietary therapy to replete these deficiencies quickly restores pancreatic exocrine function in most patients (3,44,57,62,63). Due to the typical prompt restoration of exocrine pancreatic function with dietary therapy, it seems unlikely that EPI in most patients with PCM is directly related to pancreatic morphological changes, described as pancreatic acinar atrophy which may progress to fibrosis (see reviews (44,63)). These pancreatic morphologic abnormalities in PCM resemble those in CD described at autopsy (discussed in Pancreatitis section) (1), and perhaps could account for rare instances of irreversible EPI in CD, described above. Despite these morphological similarities, PCM is unlikely to be a true independent primary cause of EPI in CD because EPI in CD is not dependent on nutritional status (10).
4. Pancreatitis and Celiac Disease
CD associates with an increased risk of developing acute pancreatitis (AP) and chronic pancreatitis (CP); the latter has a much stronger association (30,42,55). The overall risk of pancreatitis is approximately 3-fold higher than the general population (30,55). The risks (Hazards ratios) of pancreatitis appear to be higher for CP compared to AP (3.3-19.8 vs. 1.9) (30,55). As previously reviewed (15), the variability in the reported risk of pancreatitis is likely due to inaccurate diagnoses of AP and CP, which were based primarily on International Classification of Diseases-Clinical Modification (ICD-CM codes) (editions 7-10). These ICD-CM codes are only 83% accurate for definite AP in the Swedish Registry (48) and only 49% accurate for definite CP based on a study at the University of Michigan (50). Because hyperamylasemia without AP occurs in CD (7,46) and diagnosis of AP did not necessarily require imaging evidence, it is possible there were misdiagnoses of AP, which most commonly would represent over diagnosis of AP in patients with nonspecific hyperamylasemia or macroamylasemia.
Pancreatic histological findings described for CD disease are based on an autopsy study of 6 patients without documentation of clinical pancreatitis (1). Abnormalities included acinar cell atrophy in one patient and fibrosis (fine, interlobular and intralobular fibrosis) in 4 other patients. Over time, progression of these features may give rise to evidence of clinical pancreatitis in a subset of patients.
Proposed mechanisms of pancreatitis include immune-mediated pancreatic inflammation and papillary stenosis. The mechanism of immune-mediated pancreatic inflammation is poorly understood. CD has an increased prevalence of autoimmune diseases (35), but only a single case of co-existent autoimmune pancreatitis and CD has been reported (31). It has also been proposed that chronic duodenal inflammation may give rise to papillary stenosis and recurrent pancreatitis (42). Data to support this supposition is that CD was diagnosed in 7.1% of patients (12 of 169) referred to tertiary institution with suspected sphincter of Oddi dysfunction, having either recurrent abdominal pain (2 of 12) or idiopathic pancreatitis (10 of 12) (42). Although no patient had recurrent pancreatitis during the mean 22 month follow-up period, it is unclear whether treatment aimed at sphincter of Oddi dysfunction (biliary and pancreatic sphincterotomy) is helpful because all 10 patients who received this therapy also received concomitant GFD. As a word of caution about endoscopic therapy, it should be noted that in idiopathic pancreatitis, recurrent attacks of pancreatitis commonly persist despite endoscopic therapy, as recently reviewed (68).
There is likely an unrecognized incidence of CD in idiopathic pancreatitis. Approximately 10-30% of patients with AP have idiopathic pancreatitis (27). Of these, pancreatic endoscopic ultrasonography (EUS) can identify an etiology in 30-55% of cases (61,65,71). but a subset of these patients may actually have unidentified CD. Pancreatic calcifications are absent in patients from most series of CD patients, except for two patients in studies highlighted in Table 1 and Table 2 (19,43), and few additional and scattered examples of CD associated with non-alcoholic calcific chronic pancreatitis (2,33,45). In a preliminary retrospective study of patients undergoing EUS for idiopathic pancreatitis, who also had duodenal biopsy, 7.4% (5/68) of patients with definite chronic pancreatitis (by Mayo Criteria (49)) had CD (34), a 10-fold increase over the 0.71% prevalence of CD noted within the general U.S. population (54). These data mirror the 7.1 prevalence of CD in patients with suspected sphincter of Oddi dysfunction (42) and would support screening for CP as part of the evaluation of patients with idiopathic pancreatitis.
5. Treatment of Pancreatic Component of Celiac Disease
In CD, the likelihood of coexisting EPI depends on the clinical context. In routine clinical practice, accurate testing for EPI can be challenging in CD, which can be mitigated in part by a trial of empiric treatment with pancreatic enzyme replacement therapy (PERT).
As a point of emphasis it is worth reiterating that EPI is common but variable in patients with CD, ranging 0-77.8% (Table 1) but severe EPI (< 10% normal enzyme outputs evoked by CCK) sufficient to cause pancreatic steatorrhea is uncommon, ranging in prevalence from 0-18.8% in small series having a minimum of 5 patients (Table 2), with only 10 cases confirmed by direct PFTs or clinical history and autopsy. Strong clinical clues for severe EPI in CD include intractable malabsorption or weight loss despite adherence to a GFD. In patients with CD and milder symptomatology it is possible that PERT may be clinically useful, at least in children, but this is not standard of care. In a double blind randomized controlled trial PERT was useful in the first 30 days after diagnosis of CD in a pediatric population (9). For persistent malabsorption despite GFD and PERT, inadvertent gluten ingestion and refractory CD should be considered, which can be addressed with intensive counseling by an experienced registered dietician, serologic testing and repeat duodenal biopsies to exclude alterations in T lymphocyte morphology suggestive of enteropathy-associated T-cell lymphoma (EATL). An additional cause of malabsorption relevant to CD include decreased micellar concentration of bile acids because of delayed gallbladder contraction, precipitation of bile acids due to low luminal pH, deconjugation due to small intestinal bacterial overgrowth, and dilution (14,21,28,32,59). Other relevant causes include inadequate dosing or noncompliance with PERT; acid induced degradation or delayed release of PERT; biliary obstruction or ileal disease; other duodenal diseases (e.g. giardia); and a mixing disorder due to timing of PERT, gastroparesis or gastrointestinal surgery (16).
The most specific tests for detecting EPI in the contexts of CD are direct pancreatic function tests (PFT), performed by collecting and measuring pancreatic enzyme secretions into the duodenum evoked by administration of intravenous secretagogues or a standard test meal. Unfortunately, these tests are not widely available. Indirect tests of pancreatic exocrine function are more widely available and simpler to perform but less accurate. For example, 25-30% of patients with various small bowel enteropathies have a low pancreatic fecal elastase due to dilutional effects of diarrhea (4,11,37,56,,58), such that fecal elastase has been considered a general marker of enteropathy (37). For this reason, use of fecal elastase to assess pancreatic digestive function in CD is problematic, as illustrated by a recent study which reported diarrhea in 80% of 36 patients with CD, of which 28% (10 of 36) had a modestly reduced fecal elastase (mean 141.6 μg/g of stool) (47). Quantitative fecal fat testing is cumbersome and does not distinguish among specific causes of steatorrhea, but documentation of steatorrhea (a summation of multiple digestive processes) may influence decisions to offer additional testing for active CD, small intestinal bacterial overgrowth or other causes and targeted or empiric therapy for these disorders, including secondary EPI.
PERT treatment of severe EPI in patients with CD is similar to treatment of EPI related to chronic pancreatitis (13). There is no primary data to suggest that either enteric coated or uncoated formulations of PERT have greater efficacy.
Financial Support: MJD receives research support from the National Institutes of Health (DK106647).
6. References
- Adlersberg D and Schein J. Clinical and pathologic studies in sprue. J Am Med Assoc 134(17): 1459-1467,1947. PMID: 20255616
- Arya S, Rana SS, Sinha SK, Nagi B and Bhasin DK. Celiac disease and chronic calcific pancreatitis with pancreas divisum. Gastrointest Endosc 63(7): 1080-1081,2006. PMID: 16733138
- Barbezat GO and Hansen JD. The exocrine pancreas and protein-calorie malnutrition. Pediatrics 42(1): 77-92,1968. PMID: 5657699
- Beharry S, Ellis L, Corey M, Marcon M and Durie P. How useful is fecal pancreatic elastase 1 as a marker of exocrine pancreatic disease? J Pediatr 141(1): 84-90,2002. PMID: 12091856
- Benson GD, Kowlessar OD and Sleisenger MH. Adult Celiac Disease with Emphasis Upon Response to the Gluten-Free Diet. Medicine (Baltimore) 43: 1-40,1964. PMID: 14115935
- Calam J, Ellis A and Dockray GJ. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest 69(1): 218-225,1982. PMID: 7033291
- Carroccio A, Di Prima L, Scalici C, Soresi M, Cefalu AB, Noto D, et al. Unexplained elevated serum pancreatic enzymes: a reason to suspect celiac disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 4(4): 455-459,2006. PMID: 16616350
- Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, et al. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut 32(7): 796-799,1991. PMID: 1855688
- Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Greco L, et al. Pancreatic enzyme therapy in childhood celiac disease. A double-blind prospective randomized study. Dig Dis Sci 40(12): 2555-2560,1995. PMID: 8536512
- Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Soresi M, et al. Pancreatic insufficiency in celiac disease is not dependent on nutritional status. Digestive Diseases and Sciences 39(10): 2235-2242,1994. PMID: 7924748
- Carroccio A, Verghi F, Santini B, Lucidi V, Iacono G, Cavataio F, et al. Diagnostic accuracy of fecal elastase 1 assay in patients with pancreatic maldigestion or intestinal malabsorption: a collaborative study of the Italian Society of Pediatric Gastroenterology and Hepatology. Dig Dis Sci 46(6): 1335-1342,2001. PMID: 11414313
- Comfort MW, Dornberger GR and et al. External pancreatic secretion as measured by the secretin test in patients with idiopathic steatorrhoea. Gastroenterology 13(2): 135-140,1949. PMID: 18135883
- DiMagno EP and DiMagno MJ. Chronic pancreatitis: landmark papers, management decisions, and future. Pancreas 45(5): 641-650,2016. PMID: 27077713
- DiMagno EP, Go WL and Summerskill WH. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology 63(1): 25-32,1972. PMID: 5055745
- DiMagno MJ and DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol 29(5): 531-536,2013. PMID: 23852141
- DiMagno MJ and Forsmark CE. Chronic pancreatitis and small intestinal bacterial overgrowth. Pancreatology: In press,2018.
- Dreiling DA. The pancreatic secretion in the malabsorption syndrome and related malnutrition states. J Mt Sinai Hosp NY 24: 243-250,1957.
- Fernanez LB, De Paula A, Prizont R, Tiscornia OM, Braguinsky J, Carreras A, et al. Exocrine pancreas insufficiency secondary to glutenenteropathy. Am J Gastroenterol 53(6): 564-569,1970. PMID: 5421627
- Fitzgerald O, Fitzgerald P, Fennelly J, McMullin JP and Boland SJ. A Clinical Study of Chronic Pancreatitis. Gut 4: 193-216,1963. PMID: 14058261
- Ford AC, Ching E and Moayyedi P. Meta-analysis: yield of diagnostic tests for coeliac disease in dyspepsia. Aliment Pharmacol Ther 30(1): 28-36,2009. PMID: 19416130
- Fordtran JS, Rector FC, Locklear TW and Ewton MF. Water and solute movement in the small intestine of patients with sprue. J Clin Invest 46(3): 287-298,1967. PMID: 6023768
- Ihse I, Lilja P, Evander A and Skude G. Time-related enzyme concentrations in duodenal aspirates after ingestion of a test meal. Scand J Gastroenterol 12(5): 629-635,1977. PMID: 918558
- Kelly CP, Bai JC, Liu E and Leffler DA. Advances in diagnosis and management of celiac disease. Gastroenterology 148(6): 1175-1186,2015. PMID: 25662623
- Kilander AF, Hanssen LE and Gillberg RE. Secretin release in coeliac disease. Plasma secretin concentration and bicarbonate output to the duodenum after intraduodenal acid infusion in coeliac patients before and after treatment. Scand J Gastroenterol 18(6): 765-769,1983. PMID: 6669941
- Krawitt EL, Zimmerman GR and Clifton JA. Location of secretin in dog duodenal mucosa. Am J Physiol 211(4): 935-938,1966. PMID: 5926580
- Lagerlöf HO. Pancreatic function and pancreatic disease: studied by means of secretin. Acta Med Scand 128(Suppl): 1-289,1942.
- Levy MJ and Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 96(9): 2540-2555, 2001. PMID: 11569674
- Low-Beer TS, Harvey RF, Davies ER and Read AF. Abnormalities of serum cholecystokinin and gallbladder emptying in celiac disease. N Engl J Med 292(18): 961-963,1975. PMID: 1117928
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut 62(1): 43-52,2013. PMID: 22345659
- Ludvigsson JF, Montgomery SM and Ekbom A. Risk of pancreatitis in 14,000 individuals with celiac disease. Clin Gastroenterol Hepatol 5(11): 1347-1353, 2007. PMID: 17702659
- Masoodi I, Wani H, Alsayari K, Sulaiman T, Hassan NS, Nazmi Alqutub A, et al. Celiac disease and autoimmune pancreatitis: an uncommon association. A case report. Eur J Gastroenterol Hepatol 23(12): 1270-1272,2011. PMID: 21946127
- Maton PN, Selden AC, Fitzpatrick ML and Chadwick VS. Defective gallbladder emptying and cholecystokinin release in celiac disease. Reversal by gluten-free diet. Gastroenterology 88(2): 391-396,1985. PMID: 3965328
- Nanda R and Anand BS. Celiac disease and tropical calcific pancreatitis. Am J Gastroenterol 88(10): 1790-1792,1993. PMID: 8213729
- Nett AS, Wamsteker EJ and DiMagno MJ. Should Patients With Established Chronic Pancreatitis Undergo Testing for Celiac Disease? Pancreas 45(10): 1529:abstract, 2016.
- Neuhausen SL, Steele L, Ryan S, Mousavi M, Pinto M, Osann KE, et al. Co-occurrence of celiac disease and other autoimmune diseases in celiacs and their first-degree relatives. J Autoimmun 31(2): 160-165,2008. PMID: 18692362
- Newsome J. The secretin test of pancreatic function and its use in steatorrhea. Gastroenterologia 74(257),1948/49.
- Nousia-Arvanitakis S. Fecal elastase-1 concentration: an indirect test of exocrine pancreatic function and a marker of an enteropathy regardless of cause. J Pediatr Gastroenterol Nutr 36(3): 314-315,2003. PMID: 12604968
- Nousia-Arvanitakis S, Karagiozoglou-Lamboudes T, Aggouridaki C, Malaka-Lambrellis E, Galli-Tsinopoulou A and Xefteri M. Influence of jejunal morphology changes on exocrine pancreatic function in celiac disease. J Pediatr Gastroenterol Nutr 29(1): 81-85,1999. PMID: 10400109
- Novis BH, Bank S and Marks IN. Exocrine pancreatic function in intestinal malabsorption and small bowel disease. Am J Dig Dis 17(6): 489-494,1972. PMID: 5030741
- O'Connor FA, McLoughlin JC and Buchanan KD. Impaired immunoreactive secretin release in coeliac disease. Br Med J 1(6064): 811,1977. PMID: 851740
- Owyang C and Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 127(3): 957-969,2004. PMID: 15362050
- Patel RS, Johlin FC, Jr. and Murray JA. Celiac disease and recurrent pancreatitis. Gastrointestinal endoscopy 50(6): 823-827,1999. PMID: 10570344
- Pink IJ and Creamer B. Response to a gluten-free diet of patients with the coeliac syndrome. Lancet 1(7485): 300-304,1967. PMID: 4163509
- Pitchumoni CS. Pancreas in primary malnutrition disorders. Am J Clin Nutr 26(3): 374-379,1973. PMID: 4632067
- Pitchumoni CS, Thomas E, Balthazar E and Sherling B. Chronic calcific pancreatitis in association with celiac disease. The American journal of gastroenterology 68(4): 358-361,1977. PMID: 605891
- Rabsztyn A, Green PH, Berti I, Fasano A, Perman JA and Horvath K. Macroamylasemia in patients with celiac disease. Am J Gastroenterol 96(4): 1096-1100,2001. PMID: 11316153
- Rana SS, Dambalkar A, Chhabra P, Sharma R, Nada R, Sharma V, et al. Is pancreatic exocrine insufficiency in celiac disease related to structural alterations in pancreatic parenchyma? Ann Gastroenterol 29(3): 363-366,2016. PMID: 27366039
- Razavi D, Ljung R, Lu Y, Andren-Sandberg A and Lindblad M. Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology 11(5): 525-532,2011. PMID: 22094886
- Reddy N, Nangia S and DiMagno MJ. How accurate is the chronic pancreatitis (CP) ICD-9-CM (577.1) code? Pancreas 39(8): A1342 (Abstract), 2010.
- Reddy NG, Nangia S and DiMagno MJ. The Chronic Pancreatitis International Classification of Diseases, Ninth Revision, Clinical Modification Code 577.1 Is Inaccurate Compared With Criterion-Standard Clinical Diagnostic Scoring Systems. Pancreas 45(9): 1276-1281,2016. PMID: 27088491
- Regan PT and DiMagno EP. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology 78(3): 484-487,1980. PMID: 7351287
- Rhodes RA, Tai HH and Chey WY. Impairment of secretin release in celiac sprue. Am J Dig Dis 23(9): 833-839,1978. PMID: 30279
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA and American College of G. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 108(5): 656-676; quiz 677,2013. PMID: 23609613
- Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA and Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 107(10): 1538-1544; quiz 1537, 1545,2012. PMID: 22850429
- Sadr-Azodi O, Sanders DS, Murray JA and Ludvigsson JF. Patients with celiac disease have an inreased risk for pancreatitis. Clin Gastroenterol Hepatol 10(10): 1136-1142 e1133,2012. PMID: 22801059
- Salvatore S, Finazzi S, Barassi A, Verzelletti M, Tosi A, Melzi d'Eril GV, et al. Low fecal elastase: potentially related to transient small bowel damage resulting from enteric pathogens. J Pediatr Gastroenterol Nutr 36(3): 392-396,2003. PMID: 12604981
- Sauniere JF, Sarles H, Attia Y, Lombardo A, Yoman TN, Laugier R, et al. Exocrine pancreatic function of children from the Ivory Coast compared to French children. Effect of kwashiorkor. Dig Dis Sci 31(5): 481-486,1986. PMID: 3009110
- Schappi MG, Smith VV, Cubitt D, Milla PJ and Lindley KJ. Faecal elastase 1 concentration is a marker of duodenal enteropathy. Arch Dis Child 86(1): 50-53, 2002. PMID: 11806885
- Schmid WC, Phillips SF and Summerskill WH. Jejunal secretion of electrolytes and water in nontropical sprue. J Lab Clin Med 73(5): 772-783,1969. PMID: 5779262
- Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 16(6): 823-836,2018. PMID: 29551598
- Singla V and Garg PK. Role of diagnostic and therapeutic endoscopic ultrasonography in benign pancreatic diseases. Endosc Ultrasound 2(3): 134-141,2013. PMID: 24949381
- Tandon BN, Banks PA, George PK, Sama SK, Ramachandran K and Gandhi PC. Recovery of exocrine pancreatic function in adult protein-calorie malnutrition. Gastroenterology 58(3): 358-362,1970. PMID: 5437989
- Tandon BN, George PK, Sama SK, Ramachandran K and Gandhi PC. Exocrine pancreatic function in protein--calorie malnutrition disease of adults. Am J Clin Nutr 22(11): 1476-1482,1969. PMID: 5350763
- Thaysen EH, Muellertz S, Worning H and Bang HO. Amylase Concentration of Duodenal Aspirates after Stimulation of the Pancreas by a Standard Meal. Clinical Evaluation in Gastrointestinal Disorders. Gastroenterology 46: 23-31,1964. PMID: 14114718
- Thevenot A, Bournet B, Otal P, Canevet G, Moreau J and Buscail L. Endoscopic ultrasound and magnetic resonance cholangiopancreatography in patients with idiopathic acute pancreatitis. Dig Dis Sci 58(8): 2361-2368,2013. PMID: 23508982
- Weinstein LD and Herskovic T. Rectal seepage of oil in a patient with celiac disease and secondary pancreatic insufficiency. Am J Dig Dis 13: 762-765,1968.
- Weizman Z, Hamilton JR, Kopelman HR, Cleghorn G and Durie PR. Treatment failure in celiac disease due to coexistent exocrine pancreatic insufficiency. Pediatrics 80(6): 924-926,1987. PMID: 3684405
- Wilcox CM, Seay T, Kim H and Varadarajulu S. Prospective endoscopic ultrasound-based approach to the evaluation of idiopathic pancreatitis: causes, response to therapy, and long-term outcome. Am J Gastroenterol 111(9): 1339-1348,2016. PMID: 27325219
- Wormsley KG. Response to duodenal acidification in man. 3. Comparison with the effects of secretin and pancreozymin. Scand J Gastroenterol 5(5): 353-360,1970. PMID: 5455820
- Worning H, Mullertz S, Thaysen EH and Bang HO. pH and concentration of pancreatic enzymes in aspirates from the human duodenum during digestion of a standard meal in patients with intestinal disorders. Scand J Gastroenterol 2(2): 81-89,1967. PMID: 20184473
- Yusoff IF, Raymond G and Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 60(5): 673-678,2004. PMID: 15557941
- Zieve L, Silvis SE, Mulford B and Blackwood WD. Secretion of pancreatic enzymes. I. Response to secretin and pancreozymin. Am J Dig Dis 11(9): 671-684,1966. PMID: 5946356


