Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2015.34
| Attachment | Size |
|---|---|
| 178.93 KB |
1. Introduction
The interaction between diabetes mellitus (DM) and pancreatic ductal adenocarcinoma (PDAC) is complex. Although long-standing diabetes is a modest risk factor for PDAC, DM is often induced by PDAC (16). In addition, recent studies suggest that anti-diabetic medications can modify risk of PDAC in subjects with DM (24, 38). Lastly, both PDAC and DM share common risk factors such as obesity. DM secondary to an underlying disease of the exocrine pancreas is currently classified as type 3c DM by the American Diabetic Association. Recent onset DM (within 36 months) in relation to PDAC may represent a separate entity from other pancreatic diseases such as chronic pancreatitis, and will be referred to as pancreatic cancer-associated diabetes mellitus (PaCDM). The terminology of PaCDM does not technically involve DM secondary to pancreatic resection, however this is discussed in the present chapter as it relates to treatment of PDAC and provides supportive evidence for the theory that PaCDM may actually be induced by cancer.
2. Characteristics of PaCDM
Estimates of the prevalence of DM in PDAC differ depending on whether or not subjects are screened for diabetes (using fasting glucose or glucose tolerance test) or data on DM is retrospectively obtained through chart review or patient self-reporting. When evaluated by glucose tolerance testing or fasting glucose measurements, hyperglycemia occurs in up to 80% of PDAC patients at the time of diagnosis, while approximately 45-65% of PDAC patients have DM (28, 31). In a large prospective study of PDAC patients with a recent fasting blood glucose level, DM was present in 47% of patients (243/512), impaired fasting glucose in 38%, and normoglycemia in 14% (Figure 1) (28). Of those with DM, the duration of DM was <2 years for 74% (177/243), which represented 34% of the entire study population.
The clinical profile of PaCDM is not significantly different compared with those having type 2 diabetes mellitus (T2DM). The comparison of PaCDM with non-cancer controls with DM revealed similar age, gender distribution, adult body mass index, and frequency of family history of DM (28). Although the body mass index is similar at the time of DM onset, one study demonstrated most patients with T2DM are gaining weight at the time of DM onset, while many with PaCDM develop DM despite preceding weight loss (17).

Figure 1. Distribution of fasting blood glucose among pancreatic cancer cases and controls. Normal fasting glucose, ≤99 mg/dL; Impaired fasting glucose, 100-125 mg/dL; DM ≥126 mg/dL. Adapted with permission from Pannala et al (28).
3. Potential Mechanisms of PaCDM
Several explanations for PaCDM have been considered including it simply being an incidental finding, or mechanistically as the result of cancer cachexia or secondary parenchymal destruction. Additionally, there are emerging data supporting the hypothesis PaCDM may be tumor-induced, representing a paraneoplastic phenomenon.
Is DM in PDAC is Simply T2DM Ddentified Due to Intensive Testing at the Time of Diagnosis?
One explanation for PACDM is that those with PaC are more likely to have DM due to the presence of canonical risk factors for T2DM, including obesity and family history of DM. Since PDAC patients undergo extensive testing immediately preceding diagnosis, it is possible that PaCDM is simply T2DM. However, DM is not increased in prevalence in other cancers where similar testing would be undertaken, such as lung, breast, colon, and prostate cancer (Figure 2) (1). Thus, the high (50-70%) prevalence of DM at the time of PDAC diagnosis cannot be explained by incidental discovery of preexisting T2DM.
Does Insulin Resistance Due to Cancer Cachexia Precipitates PaCDM?
Both PDAC and cachexia have been associated with insulin resistance (33). It has therefore been proposed that cachexia-induced insulin resistance may unmask DM in subjects with PDAC, analogous to steroid-induced or gestational DM. However, the prevalence of DM is not increased in other cancers associated with cachexia, and the onset of PaCDM precedes onset of cachexia by many months (1, 17). Therefore, cachexia alone appears insufficient to cause DM, although it may contribute to worsening of preexisting DM.
Is DM in PDAC is Secondary to Parenchymal Destruction?
As in chronic pancreatitis, it is certainly the case that patients with large tumors can develop DM as a consequence of destruction of the islet-containing pancreatic parenchyma. Additionally, many pancreatic tumors cause pancreatic duct obstruction and atrophy of the pancreas upstream from the obstruction. Thus, obstructive chronic pancreatitis could be a potential cause of PaCDM.

Figure 2. Prevalence of DM in PDAC compared to common cancers and non-cancer controls. Adapted with permission from Aggarwal et al (1).
However, this is not a predominant factor in most cases, considering the observation that the onset of DM typically occurs 12 months prior to clinical diagnosis. Additionally, at the time of DM onset there is frequently no radiographically detectable mass or pancreatic atrophy (12, 29). Lastly, islet cell loss results in low insulin levels and decreased peripheral insulin resistance, however PDAC is associated with high insulin levels and marked insulin resistance (4, 6, 36).
Is Pancreatic Cancer-Induced DM a Paraneoplastic Event?
There are growing epidemiological data to support the hypothesis that PaCDM can be the result of a paraneoplastic process. Although long-standing DM is considered a modest risk factor for development of PDAC, there is an even greater risk in those with the onset of DM within 3-5 years of cancer diagnosis (9, 20). A population based study of more than 2,000 patients with new-onset DM reported an incidence of PaC of 0.85% (1 in 125), which was 8 times the risk of the general population (8). These results were confirmed in later studies involving patients identified through the SEER and Veterans’ Health Administration National Patient Care databases (14, 41). The markedly increased risk of PDAC in patients with new-onset DM compared to long-standing DM suggests that PDAC itself may cause DM, representing an early disease manifestation rather than a predisposing risk factor. The development of DM at an early stage in PDAC suggests new-onset DM could be used to enrich a higher risk population to target for screening of sporadic PDAC (27). However, additional tests to discriminate DM secondary to PDAC from the much more prevalent T2DM for this strategy to be effective.
Additional evidence suggesting PaCDM is a paraneoplastic phenomenon is the improvement or resolution of DM with cancer-related treatments. Although it is anticipated patients with PDAC would have increased likelihood of developing DM postoperatively, there is often paradoxical improvement in the glycemic status. While PDAC patients with long-standing DM have persistent DM following pancreatic resection, patients with PDAC and new-onset DM often experience resolution of diabetes in the postoperative setting (Figure 3) (11, 28). Additionally there is often resolution of pre-operative glucose intolerance and peripheral insulin resistance (11, 30). Similarly, in those with new-onset DM undergoing neoadjuvant chemotherapy increased tumor destruction was associated with greater odds of DM resolution (13). Although either treatment could contribute to worsened DM, the commonly observed resolution of DM favors a tumor-mediate diabetic state in some patients with PDAC.
DM occurring in the setting of PDAC is generally believed to be the result of both β-cell dysfunction and peripheral insulin resistance, however the mechanisms of disease are not fully understood. Adrenomedullin is a hormone with receptors on β-cells that has recently been proposed mediator as a of β-cell dysfunction in PaCDM (2). Adrenomedullin was demonstrated to inhibit insulin secretion when examined in both in vitro and in vivo tumor models. Additionally, plasma levels of adrenomedullin where increased in patients with PDAC compared to diabetic and non-diabetic controls, and those with PaCDM had higher levels than those with PDAC without DM. It has previously been shown that adrenomedullin is upregulated in the setting of hypoxia or hypoglycemia, suggesting this is a mediator of β-cell dysfunction in response to the PDAC tumor microenvironment (22, 26).
Other potential mediators of insulin resistance in PDAC including islet amyloid polypeptide and S-100A8 N-terminal peptide have been investigated; however, there are no compelling data in humans confirming their role (3, 5, 7). Pancreatic polypeptide (PP) is a hormone that appears to respond to stimulation differently in PDAC. This hormone normally mediates hepatic sensitivity to insulin; subcutaneous infusion of PP enhances insulin sensitivity and decreases insulin requirements (32, 39). A recent proof of concept study demonstrated a blunted PP response to mixed meal stimulation test in those with PDAC-induced DM compared to T2DM (15). Notably, the abnormal response was only observed in those with a tumor located in the head or body of the pancreas, so it is uncertain whether the decreased PP responsiveness is a consequence of mass destruction of the ventral pancreas or an alternate mediating factor.
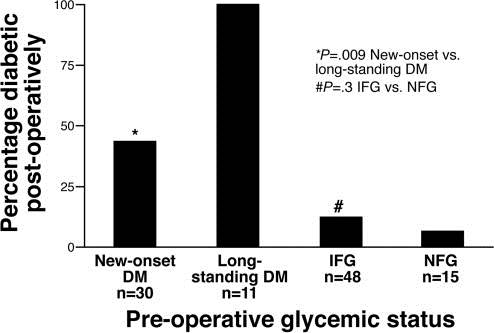
Figure 3. Prevalence of DM after pancreaticoduodenectomy for PDAC. New-onset DM was defined as a duration of ≤2 years duration in this study. Impaired fasting glucose (IFG) (100-125 mg/dL); Normal fasting glucose (NFG), ≤99 mg/dL). Adapted with permission from Pannala et al (28); with permission from Elsevier.
Thus, although a biochemical mediator of insulin resistance in PDAC remains under consideration, there is currently no direct evidence of this relationship. Another emerging possible mechanism of insulin resistance involves the interaction between PDAC and adipose tissue. Proposed pathways include adipose tissue inflammation, with resultant alterations of secreted adipokine levels (particularly adiponectin and leptin), inflammasome-mediated cytokine release (including tumor necrosis factor, IL-6, and monocyte chemoattractant protein 1), and the generation of non-esterified fatty acids (33).
DM Following Pancreatic Surgery for Pancreatic Cancer
DM onset following pancreatic resection is another important consideration. The timing of DM onset after surgery is in contrast to the previous discussions of PaCDM, which refers to DM prior to cancer treatment. DM secondary to pancreatic resection is primarily a consequence of decreased β-cell mass, and is not unique to cancer. The prevalence of post-operative DM is dependent on several factors including the type of resection, extent of resection, and whether or not there is residual disease, such as chronic pancreatitis, in the remnant pancreas. The prevalence of pancreatogenic DM following distal pancreatectomy for tumors (malignant or benign) is approximately 5-15% (10, 23, 25, 37). However, in those undergoing distal pancreatectomy for chronic pancreatitis, the risk of DM is higher (>25%) (19, 35). Despite physiologic changes favoring improved glucose tolerance, including delayed gastric emptying and weight loss, after proximal pancreatic resections (i.e., pancreaticoduodenectomy or duodenum-preserving pancreatic head resection) a higher proportion of patients develop post-operative DM compared to distal pancreatectomy. The reported prevalence of pancreatogenic DM in those undergoing surgery for chronic pancreatitis (20-50%) (21, 34, 40) remains higher than for those undergoing proximal resection for a tumor (20-30%) (10, 18).
4. Summary
The interaction between DM and PDAC is complex, and DM as a result of PDAC remains an intriguing area of interest. Many patients with PDAC develop DM shortly prior to cancer diagnosis, which is often paradoxically ameliorated by surgical resection. In combination with an increased risk for PDAC in those with new-onset DM, these data suggests the tumor itself may induce DM. There are emerging data supporting this concept of PDAC-induced paraneoplastic DM, including the identification of adrenomedullin, a potential humoral mediator of β-cell dysfunction. Further investigations into the mechanisms of β-cell dysfunction and peripheral insulin resistance in PaCDM may lead to additional insights into early detection and treatment of PDAC.
5. References
- Aggarwal G, Kamada P and Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas 42(2): 198-201, 2013. PMID: 23000893.
- Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee G, et al. Adrenomedullin is Upregulated in Patients with Pancreatic Cancer and Causes Insulin Resistance in beta Cells and Mice. Gastroenterology 143(6): 1510-1517, 2012. PMID: 22960655.
- Basso D, Greco E, Fogar P, Pucci P, Flagiello A, Baldo G, et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin Chim Acta 372(1-2): 120-128, 2006. PMID: 16678810.
- Basso D, Plebani M, Fogar P, Del Favero G, Briani G, Meggiato T, et al. Beta-cell function in pancreatic adenocarcinoma. Pancreas 9(3): 332-335, 1994. PMID: 8022755.
- Basso D, Valerio A, Seraglia R, Mazza S, Piva MG, Greco E, et al. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas 24(1): 8-14, 2002. PMID: 11741177.
- Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D and Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer 67(2): 486-493, 1991. PMID: 1985741.
- Chari ST, Klee GG, Miller LJ, Raimondo M and DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology 121(3): 640-645, 2001. PMID: 11522748.
- Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M and Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129(2): 504-511, 2005. PMID: 16083707.
- Everhart J and Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273(20): 1605-1609, 1995. PMID: 7745774.
- Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R and Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg 95(1): 85-91, 2008. PMID: 18041022.
- Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, et al. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res 14(6B): 2827-2830, 1994. PMID: 7532931.
- Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. Am J Roentgenol 182(4): 897-903, 2004. PMID: 15039161.
- Gardner TB, Hessami N, Smith KD, Ripple GH, Barth RJ, Klibansky DA, et al. The effect of neoadjuvant chemoradiation on pancreatic cancer-associated diabetes mellitus. Pancreas 43(7): 1018-1021, 2014. PMID: 25000339.
- Gupta S, Vittinghoff E, Bertenthal D, Corley D, Shen H, Walter LC, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 4(11): 1366-1372; quiz 1301, 2006. PMID: 16945591.
- Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC and Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology 15(2): 162-166, 2015. PMID: 25766398.
- Hart PA and Chari ST. Diabetes mellitus and pancreatic cancer: why the association matters? Pancreas 42(8): 1207-1209, 2013. PMID: 24152945.
- Hart PA, Kamada P, Rabe KG, Srinivasan S, Basu A, Aggarwal G, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas 40(5): 768-772, 2011. PMID: 21654538.
- Huang JJ, Yeo CJ, Sohn TA, Lillemoe KD, Sauter PK, Coleman J, et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg 231(6): 890-898, 2000. PMID: 10816633.
- Hutchins RR, Hart RS, Pacifico M, Bradley NJ and Williamson RC. Long-term results of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg 236(5): 612-618, 2002. PMID: 12409667.
- Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F and Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Brit J Cancer 92(11): 2076-2083, 2005. PMID: 15886696.
- Keck T, Wellner UF, Riediger H, Adam U, Sick O, Hopt UT, et al. Long-term outcome after 92 duodenum-preserving pancreatic head resections for chronic pancreatitis: comparison of Beger and Frey procedures. J Gastrointest Surg 14(3): 549-556, 2010. PMID: 20033344.
- Keleg S, Kayed H, Jiang X, Penzel R, Giese T, Buchler MW, et al. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int J Cancer 121(1): 21-32, 2007. PMID: 17290391.
- King J, Kazanjian K, Matsumoto J, Reber HA, Yeh MW, Hines OJ, et al. Distal pancreatectomy: incidence of postoperative diabetes. J Gastrointest Surg 12(9): 1548-1553, 2008. PMID: 18543045.
- Li D, Yeung SC, Hassan MM, Konopleva M and Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137(2): 482-488, 2009. PMID: 19375425.
- Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA and Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 229(5): 693-698; discussion 698-700, 1999. PMID: 10235528.
- Natsuizaka M, Ozasa M, Darmanin S, Miyamoto M, Kondo S, Kamada S, et al. Synergistic up-regulation of Hexokinase-2, glucose transporters and angiogenic factors in pancreatic cancer cells by glucose deprivation and hypoxia. Exp Cell Res 313(15): 3337-3348, 2007. PMID: 17651733.
- Pannala R, Basu A, Petersen GM and Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 10(1): 88-95, 2009. PMID: 19111249.
- Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM and Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 134(4): 981-987, 2008. PMID: 18395079.
- Pelaez-Luna M, Takahashi N, Fletcher JG and Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol 102(10): 2157-2163, 2007. PMID: 17897335.
- Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ and Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg 80(8): 1047-1050, 1993. PMID: 8402064.
- Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ and Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg 159(2): 101-107, 1993. PMID: 8098623.
- Rabiee A, Galiatsatos P, Salas-Carrillo R, Thompson MJ, Andersen DK and Elahi D. Pancreatic polypeptide administration enhances insulin sensitivity and reduces the insulin requirement of patients on insulin pump therapy. J Diabetes Sci Technol 5(6): 1521-1528, 2011. PMID: 22226275.
- Sah RP, Nagpal SJ, Mukhopadhyay D and Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 10(7): 423-433, 2013. PMID: 23528347.
- Sakorafas GH, Farnell MB, Nagorney DM, Sarr MG and Rowland CM. Pancreatoduodenectomy for chronic pancreatitis: long-term results in 105 patients. Arch Surg 135(5): 517-523; discussion 523-514, 2000. PMID: 10807274.
- Schoenberg MH, Schlosser W, Ruck W and Beger HG. Distal pancreatectomy in chronic pancreatitis. Dig Surg 16(2): 130-136, 1999. PMID: 10207239.
- Schwarts SS, Zeidler A, Moossa AR, Kuku SF and Rubenstein AH. A prospective study of glucose tolerance, insulin, C-peptide, and glucagon responses in patients with pancreatic carcinoma. Am J Dig Dis 23(12): 1107-1114, 1978. PMID: 367155.
- Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D and Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg 137(2): 164-168, 2002. PMID: 11822953.
- Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR and Chari ST. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol 108(4): 510-519; quiz 520, 2013. PMID: 23399556.
- Sun YS, Brunicardi FC, Druck P, Walfisch S, Berlin SA, Chance RE, et al. Reversal of abnormal glucose metabolism in chronic pancreatitis by administration of pancreatic polypeptide. Am J Surg 151(1): 130-140, 1986. PMID: 3946744.
- Traverso LW and Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg 226(4): 429-435; discussion 435-428, 1997. PMID: 9351711.
- Wang F, Gupta S and Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev 15(8): 1458-1463, 2006. PMID: 16896032.


