Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2013.15
| Attachment | Size |
|---|---|
| 443.35 KB |
Introduction
Patients with autoimmune pancreatitis (AIP) often present with vague abdominal pain, jaundice, or weight loss. Differentiating AIP from pancreatic cancer is important to avoid unnecessary surgery or invasive intervention. When CT or MR reveals characteristic imaging findings of AIP, it is not difficult to make a correct diagnosis. However, differentiating AIP from pancreatic carcinoma on CT or MR can be very difficult at times. AIP is one of the most common benign conditions for which pancreatic resection is performed for suspected pancreatic carcinoma (5, 29). Combinations of ancillary findings may lead to correct diagnosis; therefore, it is important to be familiar with the various imaging findings of AIP.
Features of Autoimmune Pancreatitis
Pancreatic Parenchymal Morphology
Diffuse parenchymal enlargement of pancreas is the characteristic feature of AIP seen in 24-73% of patients (Figure 1) (3, 8, 21-23). The pancreatic border becomes featureless with effacement of the lobular contour of the pancreas, resulting in the so-called “sausage shaped pancreas” (21). Focal, mass-like enlargement of the pancreas is seen in 18-40% of patients with AIP (21-23, 28). Any portion of the pancreas can be involved, but involvement of the pancreatic head is more common (15, 22). The enlarged segment of the pancreas is typically iso-attenuating compared to the non-enlarged segment of pancreatic parenchyma (21). In a small number of cases, the focally enlarged segment forms a low-attenuation mass and may be indistinguishable from pancreatic cancer (21, 23, 27, 28). The demarcation between the normal parenchyma tends to be sharp in such cases (27). Atrophy of the pancreas upstream to the focally involved area is uncommon in patients with AIP as opposed to patients with pancreatic carcinoma. The pancreas may exhibit a long segment of low attenuation without mass-like enlargement. Rarely AIP may present as multiple low-attenuation lesions (9). Finally, the pancreas may appear normal in size or atrophic in 9-36% of patients (3, 22, 23). A normal size pancreas may result from a milder form of disease, but in such cases the enhancement pattern is usually altered (22). Pancreatic atrophy usually represents a late burnt-out phase of the disease or post-treatment state (21).
Capsule Sign
A capsule-like rim (Figure 1) is a highly specific sign of AIP, and can been seen in 14-48% of patients with AIP (8, 21-23). The capsule-like rim is low attenuation on contrast-enhanced CT, and hypo-intense on both T1- and T2-weighted images, and shows delayed enhancement on MR. The rim may diffusely surround the entire pancreas or only focal regions (22). The rim is thought to represent peripancreatic extension of the characteristic inflammatory cell infiltration (8). This is contrary to the high-attenuation rim which can be sometimes seen in infiltrating pancreatic carcinoma (2).

The high-attenuation rim represents compressed normal parenchyma by carcinoma.
Pancreatic Parenchymal Enhancement
The enhancement pattern of the pancreatic parenchyma should be carefully evaluated on CT or MR using multi-phasic technique, because it often gives a helpful clue to the diagnosis. The involved segment(s) of the pancreas commonly demonstrate delayed enhancement in AIP (8). CT attenuation of the pancreas in AIP is similar or higher than that of the liver and lower than that of spleen during the pancreatic phase, and is similar or higher than that of the liver and higher than that of spleen in hepatic phase on a biphasic CT scan (15, 30). Quantitatively, one study showed that the mean CT attenuation value of the pancreatic parenchyma in AIP was significantly lower than in normal controls during the pancreatic phase (AIP: 85 HU, normal pancreas: 104 HU; p < 0.05), but not significantly different in the hepatic phase (AIP: 96 HU, normal pancreas: 89 HU; p = 0.6) (24). Similar enhancement patterns were observed on MR (14, 20).
A similar enhancement pattern is also seen in patients with focal AIP; decreased enhancement during the pancreatic phase with delayed enhancement during the hepatic phase. On the other hand, pancreatic carcinoma commonly shows decreased enhancement in the pancreatic phase with a minimal change in the enhancement in the hepatic phase. Wakabayashi et al. evaluated the CT enhancement pattern in 9 patients with focal AIP (28). Of the 9 patients, 6 lesions were hypo-attenuating in the early phase but all were homogeneously iso-attenuating in the delayed phase. Conversely, only 2 of 80 patients with pancreatic carcinoma had homogeneous enhancement in the delayed phase. A different study showed that the mean CT attenuation value of focal AIP was not significantly different in the pancreatic phase (AIP: 71 HU, carcinoma: 59 HU; p = 0.06), but was significantly higher than carcinoma in the hepatic phase (AIP: 90 HU, carcinoma: 64 HU; p < 0.001) (24). Delayed enhancementof the mass or focally enlarged segment, defined as a 15-HUor greater increase from the pancreatic phase to the hepaticphase, was found in 7 of the 13 patients with focalAIP (54%) and in 5 of 33 patients(15%) with carcinoma (p = 0.02) (24).
MR features of autoimmune pancreatitis
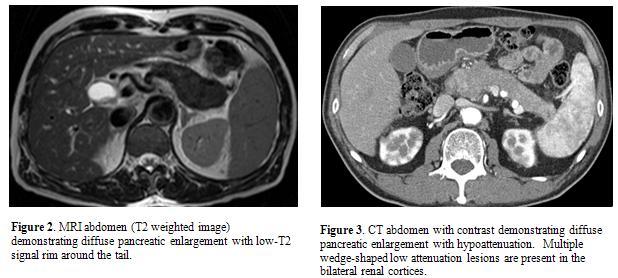
On MR, the pancreas is diffusely hypointense on T1-weighted images and slightly hyperintense on T2-weighted images (Figure 2) (8, 14, 20, 21). Enhancement characteristics on MR are similar to that seen on CT. Diffusion-weighted MR has been shown to be helpful in differentiating AIP from pancreatic cancer. Kamisawa et al showed apparent diffusion coefficient values were significantly lower in AIP (1.01 +/- 0.11 x 10-3 mm2/s) than in pancreatic cancer (1.25 +/- 0.11 x 10-3 mm2/s) and normal pancreas (1.49 +/- 0.16 x 10-3 mm2/s) (P<0.001) (11). Taniguchi et al showed that apparent diffusion coefficient values were significantly lower in AIP (0.97 +/- 0.18 x 10-3 mm2/s) compared to other types of chronic pancreatitis (1.45 +/- 0.10 x 10-3 mm2/s) (26). In addition, diffusion weighted MR was helpful in reclassifying what appeared to be focal mass-forming AIP to diffuse AIP by showing diffusely decreased apparent diffusion coefficient values in the non-enlarged pancreatic segment.
Pancreatic Ductal Imaging
Diffuse or segmental narrowing of the main pancreatic duct is the characteristic ERCP finding of AIP (ERCP Features of Autoimmune Pancreatitis) (6, 21). Diffuse narrowing of the duct is often difficult to differentiate from a normal caliber duct on CT or MR. Segmental narrowing of the main pancreatic duct may be seen as a poorly visualized segment on CT or MRCP compared to a normal caliber pancreatic duct in uninvolved segments of pancreas (12, 19). Mild pancreatic ductal dilation may be present upstream to the narrowed segment. The degree of main pancreatic duct dilation is usually milder than that seen in cases of pancreatic carcinoma. A relatively specific main pancreatic duct change of AIP is multifocal narrowing, and this may be depicted on CT or MRCP (1, 19). Although it is helpful if classic abnormalities are present, MRCP often does not provide adequate visualization of the main pancreatic duct, and is thus not considered as a satisfactory means of pancreatic ductal imaging in the current diagnostic criteria (Diagnosis of Autoimmune Pancreatitis). The duct penetrating sign, visible duct within a mass, may be helpful in differentiating AIP from pancreatic cancer (7, 16). Secretin stimulated MRCP may enhance detection of pancreatic duct penetrating sign (1). Enhancement of the pancreatic duct wall may be present in patients with AIP on portal phase or delayed phase CT (13, 22).
Miscellaneous pancreatic findings
Pancreatic pseudocysts and/or calcifications are typically associated with alcohol-induced chronic pancreatitis (28). However, calcifications are seen in 14-32% and cysts are seen in 10-12% of patients with AIP (22, 23), especially in the late or post-acute phase; therefore, presence of calcifications or cysts should not exclude the possibility of AIP (17, 18). Vessels are commonly involved by the extension of peripancreatic soft tissue in patients with AIP (44-68%). Involved veins are often narrowed, but occlusion may also occur (22).
Extra-pancreatic involvement in the abdomen
The most common site of extra-pancreatic involvement is the biliary tree presenting with asymptomatic liver test abnormalities or jaundice (23). On imaging, biliary involvement commonly appears as multifocal biliary strictures similar to primary sclerosing cholangitis. On CT or MR, the strictured bile duct commonly appears as diffuse or focal thickening of the wall. Rarely, it may form a mass which mimics cholangiocarcinoma. The kidneys are also commonly involved (25). On CT or MR, renal lesions are commonly bilateral and multiple, predominantly involving renal cortex (Figure 3). Renal parenchymal lesions can be classified as small peripheral cortical nodules, round or wedge-shaped lesions, and diffuse patchy involvement. Renal lesions may present as a large solitary mass which mimics primary renal neoplasm. Retroperitoneal fibrosis is seen in 10% of cases (Figure 4).

Differences in type 1 and type 2 AIP
A recent study by Deshpande et al (4) showed that pancreatic tail cut-off sign was only seen in type 2 AIP (4/10). Other imaging features such as the type of pancreatic swelling, presence of capsule-like rim, and common bile duct strictures were not helpful in distinguishing the two types. An international multicenter survey showed that diffuse swelling of pancreas was more common in type 1 compared to type 2 AIP (40% vs 25%) (10). The pattern of extrapancreatic organ involvement is distinct between the two types and helpful when present (10). Biliary or renal involvement and retroperitoneal fibrosis are exclusively seen in type 1 AIP, whereas inflammatory bowel disease is commonly associated with type 2 AIP (10).
Summary
Imaging features of CT and MR are critical for establishing the diagnosis of AIP and excluding other potential etiologies, particularly pancreatic cancer. Classic imaging features which are relatively specific for AIP include diffuse pancreatic enlargement, presence of a hypoattenuating capsule rim, and delayed parenchymal enhancement. Although not always present, findings of multifocal narrowing of the main pancreatic duct or other organ involvement such as biliary strictures, renal involvement, and retroperitoneal fibrosis are helpful clues for AIP diagnosis. The imaging variants of AIP (focal and multifocal involvement) are sometimes indistinguishable from malignancy and require careful evaluation for collateral diagnostic evidence.
References
- Carbognin G, Girardi V, Biasiutti C, Camera L, Manfredi R, Frulloni L, Hermans JJ, and Mucelli RP. Autoimmune pancreatitis: imaging findings on contrast-enhanced MR, MRCP and dynamic secretin-enhanced MRCP. Radiol Med 114: 1214-1231, 2009. PMID: 19789959
- Choi YJ, Byun JH, Kim JY, Kim MH, Jang SJ, Ha HK, and Lee MG. Diffuse pancreatic ductal adenocarcinoma: characteristic imaging features. European journal of radiology 67: 321-328, 2008. PMID: 17766075
- Church NI, Pereira SP, Deheragoda MG, Sandanayake N, Amin Z, Lees WR, Gillams A, Rodriguez-Justo M, Novelli M, Seward EW, Hatfield AR, and Webster GJ. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 102: 2417-2425, 2007. PMID: 17894845
- Deshpande V, Gupta R, Sainani N, Sahani DV, Virk R, Ferrone C, Khosroshahi A, Stone JH, and Lauwers GY. Subclassification of autoimmune pancreatitis: a histologic classification with clinical significance. Am J Surg Pathol 35: 26-35, 2011. PMID: 21164284
- Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH, Cameron JL, and Leach SD. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg 237: 853-858, 2003. PMID: 12796582
- Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, and Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc 55: 494-499, 2002. PMID: 11923760
- Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, and Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology 221: 107-116, 2001. PMID: 11568327
- Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, Jimi M, Sumii T, and Masuda K. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol 170: 1323-1327, 1998. PMID: 9574610
- Kajiwara M, Kojima M, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Hasebe T, Ochiai A, and Kinoshita T. Autoimmune pancreatitis with multifocal lesions. J Hepatobiliary Pancreat Surg 15: 449-452, 2008. PMID: 18670850
- Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J, Pickartz T, Lohr M, Schneider A, Frulloni L, Webster GJ, Reddy DN, Liao WC, Wang HP, Okazaki K, Shimosegawa T, Kloeppel G, and Go VL. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 40: 809-814, 2011. PMID: 21747310
- Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N, and Sasaki T. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol 105: 1870-1875, 2010. PMID: 20216538
- Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, and Kamata N. Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis? Abdom Imaging 34: 381-384, 2009. PMID: 18437450
- Kawai Y, Suzuki K, Itoh S, Takada A, Mori Y, and Naganawa S. Autoimmune pancreatitis: assessment of the enhanced duct sign on multiphase contrast-enhanced computed tomography. European journal of radiology 81: 3055-3060, 2012. PMID: 22613506
- Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, and Pozzi Mucelli R. Autoimmune Pancreatitis: Pancreatic and Extrapancreatic MR Imaging-MR Cholangiopancreatography Findings at Diagnosis, after Steroid Therapy, and at Recurrence. Radiology 260: 428-436, 2011. PMID: 21613442
- Manfredi R, Graziani R, Cicero C, Frulloni L, Carbognin G, Mantovani W, and Mucelli RP. Autoimmune pancreatitis: CT patterns and their changes after steroid treatment. Radiology 247: 435-443, 2008. PMID: 18430876
- Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Tsukamoto T, Fatima Z, and Araki T. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. Journal of magnetic resonance imaging : JMRI 35: 827-836, 2012. PMID: 22069025
- Muraki T, Hamano H, Ochi Y, Arakura N, Takayama M, Komatsu K, Komiyama Y, Kawa S, Uehara T, and Kiyosawa K. Corticosteroid-responsive pancreatic cyst found in autoimmune pancreatitis. J Gastroenterol 40: 761-766, 2005. PMID: 16082595
- Nishimura T, Masaoka T, Suzuki H, Aiura K, Nagata H, and Ishii H. Autoimmune pancreatitis with pseudocysts. J Gastroenterol 39: 1005-1010, 2004. PMID: 15549456
- Park SH, Kim MH, Kim SY, Kim HJ, Moon SH, Lee SS, Byun JH, Lee SK, Seo DW, and Lee MG. Magnetic resonance cholangiopancreatography for the diagnostic evaluation of autoimmune pancreatitis. Pancreas 39: 1191-1198, 2010. PMID: 20467343
- Rehnitz C, Klauss M, Singer R, Ehehalt R, Werner J, Buchler MW, Kauczor HU, and Grenacher L. Morphologic patterns of autoimmune pancreatitis in CT and MRI. Pancreatology : official journal of the International Association of Pancreatology 11: 240-251, 2011. PMID: 21625195
- Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, and Simeone JF. Autoimmune pancreatitis: imaging features. Radiology 233: 345-352, 2004. PMID: 15459324
- Suzuki K, Itoh S, Nagasaka T, Ogawa H, Ota T, and Naganawa S. CT findings in autoimmune pancreatitis: assessment using multiphase contrast-enhanced multisection CT. Clin Radiol 65: 735-743, 2010. PMID: 20696301
- Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, and Chari ST. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol 190: 280-286, 2008. PMID: 18212210
- Takahashi N, Fletcher JG, Hough DM, Fidler JL, Kawashima A, Mandrekar JN, and Chari ST. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR Am J Roentgenol 193: 479-484, 2009. PMID: 19620446
- Takahashi N, Kawashima A, Fletcher JG, and Chari ST. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology 242: 791-801, 2007. PMID: 17229877
- Taniguchi T, Kobayashi H, Nishikawa K, Iida E, Michigami Y, Morimoto E, Yamashita R, Miyagi K, and Okamoto M. Diffusion-weighted magnetic resonance imaging in autoimmune pancreatitis. Japanese journal of radiology 27: 138-142, 2009. PMID: 19412681
- Van Hoe L, Gryspeerdt S, Ectors N, Van Steenbergen W, Aerts R, Baert AL, and Marchal G. Nonalcoholic duct-destructive chronic pancreatitis: imaging findings. AJR Am J Roentgenol 170: 643-647, 1998. PMID: 9490945
- Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, and Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol 98: 2679-2687, 2003. PMID: 14687817
- Yadav D, Notahara K, Smyrk TC, Clain JE, Pearson RK, Farnell MB, and Chari ST. Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clin Gastroenterol Hepatol 1: 129-135, 2003. PMID: 15017505
- Yang DH, Kim KW, Kim TK, Park SH, Kim SH, Kim MH, Lee SK, Kim AY, Kim PN, Ha HK, and Lee MG. Autoimmune pancreatitis: radiologic findings in 20 patients. Abdom Imaging 31: 94-102, 2006. PMID: 16333694


