Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2020.13
I. Cyclic Nucleotides and their Biosynthesis
Cyclic nucleotides, like other nucleotides, are composed of three functional groups: a ribose sugar, a nitrogenous base, and a single phosphate group. There are two types of nitrogenous bases: purines (adenine and guanine) and pyrimidines (cytosine, uracil and thymine). A cyclic nucleotide, unlike other nucleotides, has a cyclic bond arrangement between the ribose sugar and the phosphate group. There are two main groups of cyclic nucleotides: the canonical or well-established and the non-canonical or unknown-function cyclic nucleotides. The two well-established cyclic nucleotides are adenosine-3’,5’-cyclic monophosphate (cyclic AMP) and guanine-3’,5’-cyclic monophosphate (cyclic GMP). Both cyclic AMP and cyclic GMP are second messengers. The non-canonical cyclic nucleotides include the purines inosine-3’,5’-cyclic monophosphate (cyclic IMP), xanthosine-3’,5’-cyclic monophosphate (cyclic XMP) and the pyrimidines cytidine-3’,5’-cyclic monophosphate (cyclic cCMP), uridine-3’,5’-cyclic monophosphate (cyclic UMP), and thymidine-3’,5’-cyclic monophosphate (cTMP) (145). An overview of the non-canonical cyclic nucleotides is provided in Section V.
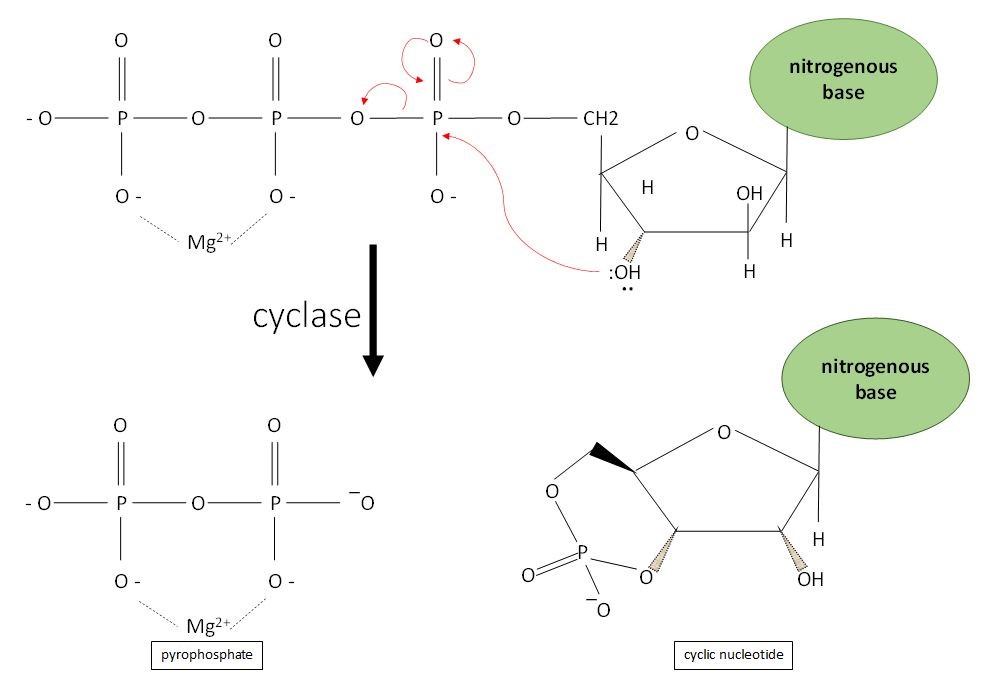
A cyclase enzyme (lyase) catalyzes the formation of the cyclic nucleotide from its nucleotide triphosphate precursor (Figure 1). Cyclic nucleotides form when the phosphate group of the molecule of nucleotide triphosphate (ATP or GTP) is attacked by the 3’ hydroxyl group of the ribose, forming a cyclic 3’,5’-phosphate ester with release of pyrophosphate. This cyclic conformation allows cyclic nucleotides to bind to proteins to which other nucleotides cannot. The reaction is an intramolecular nucleophilic reaction and requires Mg2+ as a cofactor, whose function is to decrease the overall negative charge on the ATP by complexing with two of its negatively charged oxygen. If its negative charge is not reduced, the nucleotide triphosphate cannot be approached by a nucleophile, which is, in this reaction, the 3’ hydroxyl group of the ribose (183). Soluble AC prefers Ca2+ to Mg2+ as the coenzyme to coordinate ATP binding and catalysis (154).
II. Canonical Cyclic Nucleotide Signaling in the Exocrine Pancreas
Cyclic nucleotide signaling can be initiated by two general mechanisms. One mechanism is the binding of an extracellular ligand to a transmembrane G-protein-coupled receptor (GPCR). The receptor protein has seven transmembrane α-helices connected by alternating cytosolic and extracellular loops. The N-terminus is located in the extracellular space, while the C-terminus is located in the cytosol. The ligand-binding site is in the extracellular domain and the cytosolic domain has a heterotrimeric G protein-binding site (127). After a ligand binds to the GPCR, it activates a heterotrimeric G-protein, which is composed of three subunits: a guanine nucleotide binding α-subunit, and a βγ-heterodimer (98). Depending on which family the G protein is, it goes on to activate (Gαs protein subunit) or inhibit (Gαi protein subunit) the membrane-bound cyclase.
Figure 1. Two aspartic acid residues in the active site of the cyclase (AC or GC) promotes the binding of ATP. Two Mg2+ ions are required to decrease the overall negative charge on the ATP by complexing with two of its negatively charged oxygen. Mg2+ is involved in the deprotonation of the 3’hydroxyl group in the ribose ring of ATP. Soluble AC uses Ca2+ rather than Mg2+ as a coenzyme. This step is necessary for the nucleophilic catalysis on the 5’ α-phosphate by the newly formed oxyanion. The end products of this catalytic reaction are a cyclic nucleotide (cyclic AMP or cyclic GMP) and a pyrophosphate group.
The second signaling mechanism involves the binding of a small signaling molecule to a soluble cyclase. The signal source can be either extracellular, such as nitric oxide (NO) (114), or intracellular, such as bicarbonate (189). The signaling by an extracellular ligand is limited by its ability to cross the plasma membrane. In the cytosol, the signal molecule binds to the heme-binding domain of the soluble cyclase. The cyclase, in turn, increases the intracellular levels of cyclic nucleotides (83, 153).
In the exocrine pancreas, adenylyl cyclases can be activated by either extracellular or intracellular signals. The extracellular signals can be a neurotransmitter, such as vasoactive intestinal polypeptide (VIP), a hormone, such as secretin (163), or a gas, such as NO (181). Intracellular signals include HCO3- (84). The increase in the cyclic nucleotide levels modifies the activity of downstream effectors such as kinases (64, 161), guanine-nucleotide-exchange factor (GEF) (35),RNA-binding protein (54), ion channels (77) and phosphodiesterases (30),which are discussed later in this chapter.
III. Adenylyl Cyclase/Cyclic AMP Signaling
Cyclic AMP is formed from cytosolic ATP by the enzyme adenylyl cyclase. There are ten isoforms of adenylyl cyclases; nine are anchored in the plasma membrane, with its catalytic portion protruding into the cytosol, and one is soluble (160).
A. Transmembrane AC
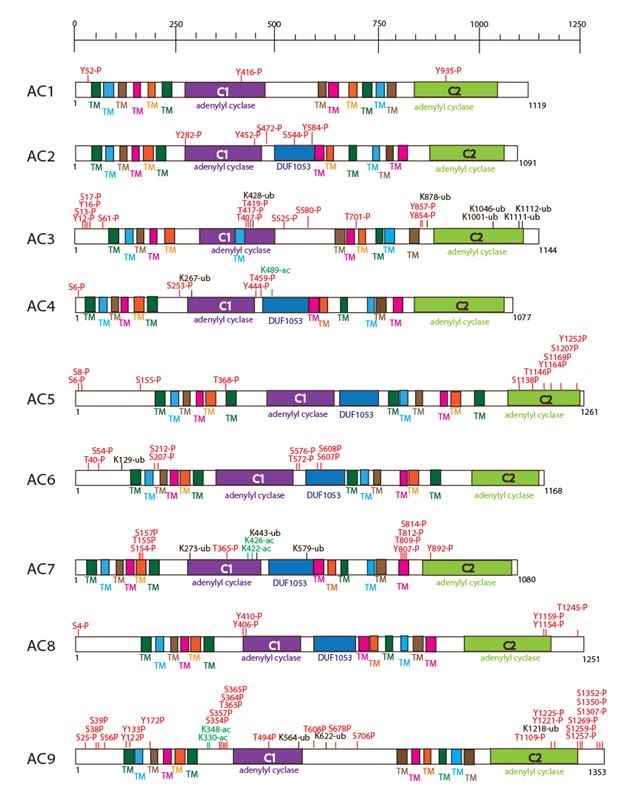
The nine transmembrane AC isoforms are each coded by a different gene (Figure 2). The human ADCY1 gene is located on chromosome 7 at p12.3, human ADCY2 gene on chromosome 5 at p15.3, human ADCY3 gene on chromosome 2 at p23.3, human ADCY4 gene on chromosome 14 at q12, human ADCY5 gene on chromosome 3 at q21.1, human ADCY6 gene on chromosome 12 at q12-q13, human ADCY7 gene on chromosome 16 at q12.1, human ADCY8 gene on chromosome 8 at q24, human ADCY9 gene on chromosome 16 at p13.3 (128). All of transmembrane AC isoforms share a high sequence homology in the primary structure of their catalytic site and the same three-dimensional structure. The AC structure is a pseudodimer that can be divided in two main regions, transmembrane and cytoplasmic regions, and further divided into five different domains: 1) the NH2 terminus, 2) the first transmembrane cluster (TM1), 3) the first cytoplasmic loop composed of C1a and C1b, 4) the second transmembrane cluster (TM2) with extracellular N-glycosylation sites, and 5) the second cytoplasmic loop composed of C2a and C2b. The transmembrane regions (TM1 and TM2), whose function is to keep the enzyme anchored in the membrane, are composed of twelve membrane-spanning helices, consist of 6 membrane-spanning helices each, with short inter-helical loops. The cytoplasmic regions C1 and C2 are approximately 40 kDa each and can be further subdivided into C1a, C1b, C2a, and C2b. Both C1a and C2a are highly conserved catalytic ATP-binding regions (31), which dimerize to form a pseudosymmetric enzyme, which forms the catalytic site. ATP binds at one of two pseudosymmetric binding sites of the C1-C2 interface. Two amino acid residues, Asn1025 and Arg1029 of AC2 are conserved among the C2 domains and critical for the catalytic activity of AC; mutation of either residue causes in a 30-100-fold reduction in the AC activity (178). A second C1 domain subsite includes a P-loop that accommodates the nucleotide phosphates and two conserved acid residues that bind to ATP through interaction with two Mg2+; one Mg2+ contributes to catalysis, whereas the second one interacts with nucleotide β- and γ-phosphates from substrate binding and possibly also for leaving-group stabilization. Both C2a and C2b are less conserved than the C1 domain (31, 88). The C1b domain is the largest domain, contains several regulatory sites and has a variable structure across the isoforms. However, the C2b domain is essentially non-existent in many isoforms, and has not yet been associated with a function (186). The overall domain structure of each human transmembrane AC isoform is shown in Figure 2 and a detailed comparison of the cytoplasmic domains (C1 and C2), transmembrane segments, acetylation, phosphorylation and ubiquitination sites of each isoform is indicated. Figure 3 shows the three dimensional model of AC and its relation to heterotrimeric G protein α subunit.
Figure 2. Schematic representation of the domain structure of the 9 human transmembrane AC isoforms. The number of amino acid residues is reported on the side of each structure. Modification sites and domains are represented with different color. The transmembrane AC isoforms share a common structure composed of two cytosolic domains (C1 and C2) and 6-transmembrane segments organized in two tandem repeats. Both C1 and C2 domains contribute to ATP binding and formation of the catalytic core. Abbreviations: TM (transmembrane segments); DUF (domain of unknown function); ac (acetylation); P (phosphorylation site); ub (ubiquitination); S (serine); K (lysine); T (threonine); Y (tyrosine). (Data obtained from PhosphoSitePlus).
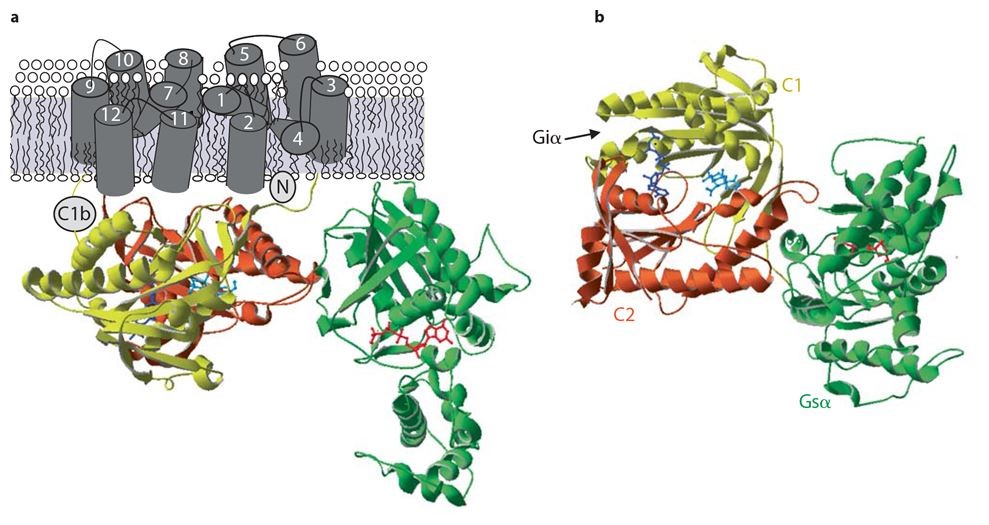
Figure 3. Crystal structure of Adenylyl Cyclase. (a) The figure shows the catalytic domains of mammalian AC C1 (yellow) and C2 (rust) with Gαs (green). The location of forskolin (cyan) and P-site inhibitor (dark blue) is also shown. (b) An alternate view from cytoplasmic side, showing forskolin and catalytic site. The interaction site of Giα with C1 domain is indicated by an arrow. This figure was reproduced with permission from (139).
Without stimulation, the enzyme AC is constitutively inactive. There are at least two heterotrimeric G-proteins responsible for the regulation of transmembrane AC activity: Gs and Gi. When a secretagogue (for example: secretin, vasoactive intestinal polypeptide) binds to its GPCR, it causes a change in the conformation of the receptor that stimulates the Gsα subunit to exchange GDP against GTP, which causes GTP-Gsα to detach from the Gβγ subunits and bind to the two cytoplasmic regions of transmembrane AC (43). With GTP-Gsα bound, AC becomes active and converts ATP to cyclic AMP in a process involving release of water and a pyrophosphate. Gsα has shown to play an important role in the exocrine pancreas and Gsα-deficient mice show morphological changes in the exocrine pancreas, as well as malnutrition and dehydration (174). Certain isoforms of transmembrane ACs are also positively (AC2, AC4, AC5, AC6, AC7) or negatively (AC1, AC3, AC8) regulated by the Gβγ subunits, which also bind to the two cytoplasmic regions of transmembrane AC (43).
When a GPCR is coupled to the heterotrimeric protein Gi, GTP-Giα binds to adenylyl cyclase and, unlike GTP-Gsα, GTP-Giα inhibits the activity of the enzyme, causing lower levels of cyclic AMP in the cells. In the pancreas, somatostatin binds to its SS2 receptor and causes activation of Giα subunit and inhibition of adenylyl cyclase (109, 151). Once the concentration of the ligand is below activation levels, the Gα subunit, which has an intrinsic GTPase activity, hydrolyzes GTP to GDP, re-associates with Gβγ and becomes inactive. The cycle of GTP hydrolysis and inactivation occur within seconds after the G protein has been activated. Upon inactivation, G proteins are ready to be reactivated by another extracellular signal.
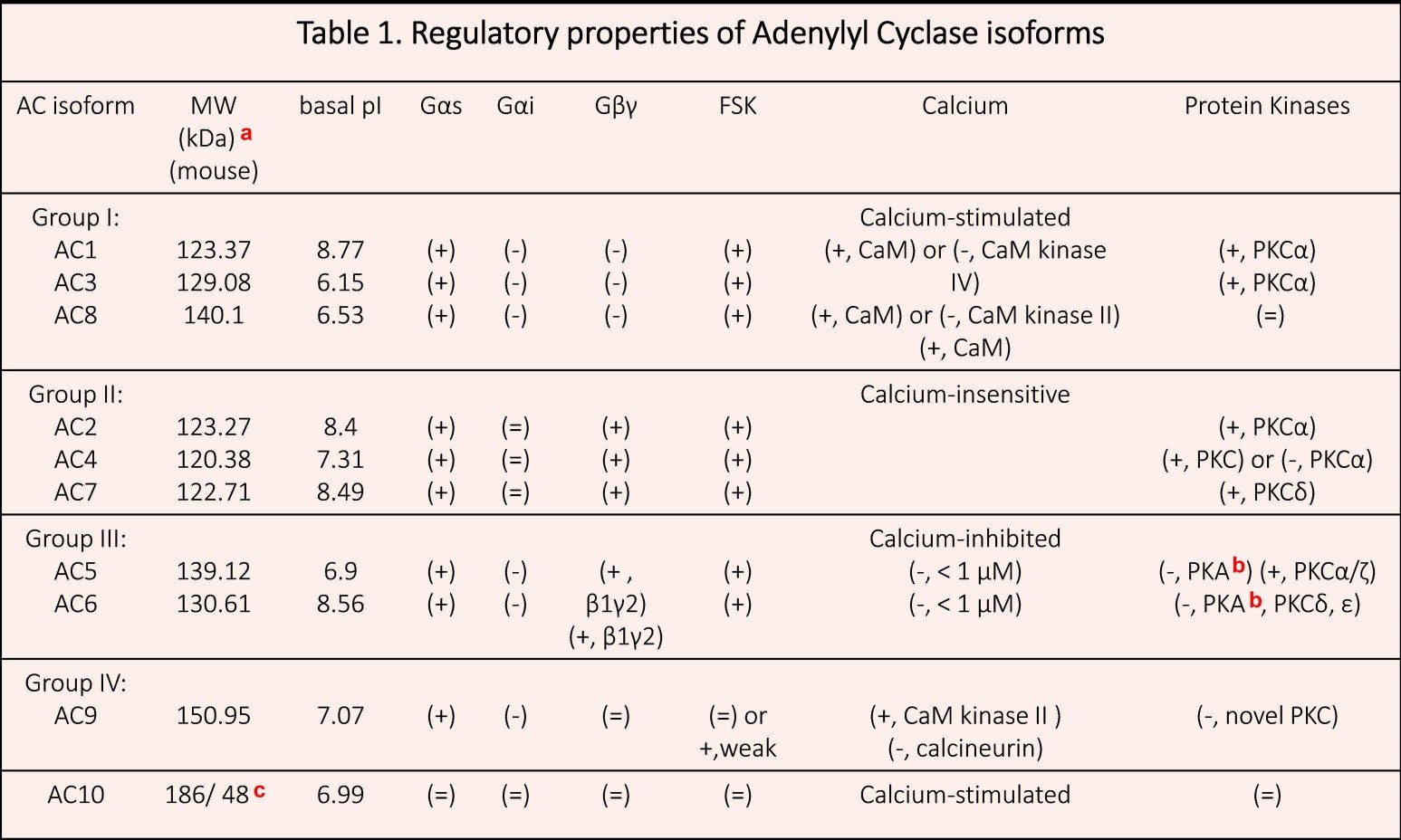
Transmembrane ACs are classified into four groups based on their regulatory properties (Table 1 and Figure 4):
- Group I, which consists of Ca2+-stimulated isoforms: AC1, AC3, AC8.
- Group II, which consists of Gβγ-stimulated isoforms: AC2, AC4, AC7.
- Group III, which consists of Gαi/Ca2+-inhibited isoforms: AC5, AC6.
- Group IV, which solely consists of Ca2+-, Gβγ-insensitive isoform: AC9.
Table 1. Regulatory properties of AC isoforms. (+) AC activity is stimulated; (-) AC activity is inhibited; (=) AC activity is not modified. Data taken from (14, 36, 116, 170). (a) The molecular weight (MW) and basal isoelectric point (pI) data was obtained from PhosphoSitePlus (Cell Signaling Technology, Inc.) (b) AC6 is directly phosphorylated by PKA at Ser674, and thereby inhibited (28). (c)186 kDa (the full length form), and 48 kDa (the truncated form).
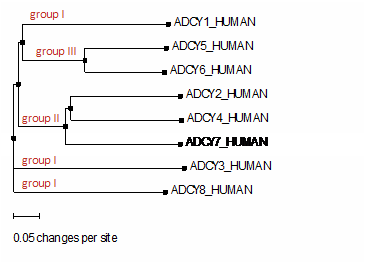
Figure 4. Phylogenetic tree of the transmembrane AC sequence. A dendrogram shows the degree of relatedness of sequence of transmembrane AC isoforms built by ClustalW. The length of each horizontal line indicates estimated evolutionary distance. Branches separate an individual subfamily. Groups defined by differences in regulatory properties are indicated in red. The order of evolution as followed (from the most conserved to the less conserved): ADCY3 > ADCY1> ADCY7 >ADCY4. ADCY5 and ADCY6, as well as ADCY2 and ADCY4 are co-evolutionary isoforms.
The expression profile of the transmembrane AC isoforms in intact mouse pancreas, isolated pancreatic acini and duct fragment has been established using RT-PCR. Five different transmembrane AC isoforms were identified in pancreatic exocrine cells: AC3, AC4, AC6, AC9 mRNAs were expressed in isolated pancreatic acini and sealed duct fragments, whereas AC7 mRNAs was only expressed in duct fragments (133). Using real-time quantitative PCR analysis, the relative expression of each isoform in pancreatic acini and ducts compared to the intact pancreas was assessed: isolated pancreatic acini were shown to have higher transcript levels of AC6 compared with intact pancreas, whereas isolated duct fragments were shown to have higher transcript levels of AC4, AC6 and AC7 compared with intact pancreas.
Similar transcript levels of AC3 and AC9 were observed in pancreas, acini and ducts (133). In conclusion, several adenylyl cyclase isoforms are expressed in pancreatic exocrine cells, with AC6 being highly expressed in both pancreatic acinar and duct cells.
B. Soluble AC
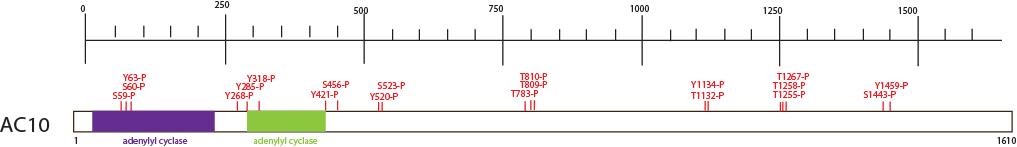
Soluble AC10, is unique in some many ways. The human ADCY10 gene is located on chromosome 1 at q24. It not anchored in the plasma membrane. As indicated in the Figure 5, the catalytic domains C1 and C2 are located at the N-terminus and connected by a ~68 residue linker that forms a death domain-like subdomain with the ~33 residue N-terminus of the protein. The C-terminal from this C1-C2 tandem of the full-length mammalian soluble AC comprises a ~1100 residue C-terminal region without a transmembrane region (81). Its catalytic domain sequence is more closely related to some bacterial ACs than mammalian ACs (14). Unlike transmembrane AC, soluble AC has no transmembrane domain. For that reason, its location is in the cytosol, but can be associated with certain cellular organelles, such as the nucleus, mitochondria and microtubules (188). Unlike transmembrane ACs which are regulated by G proteins, forskolin and calmodulin among others, soluble AC is stimulated by HCO3- (189). The HCO3- ion induces a conformational change of the active site of soluble AC similar to that observed in transmembrane ACs upon stimulation with Gαs (154). Using RT-PCR and Western-blotting, soluble AC has also been identified in acinar cells. By immunohistochemistry using a soluble AC antibody, AC10 has been localized just below the apical region of the cell in non-stimulated condition and, after treatment with the CCK analog caerulein, a punctuate intracellular pattern was seen (84).
Figure 5. Schematic representation of the domain structure of the soluble AC isoform, AC10. The number of amino acid residues is reported on the side of structure. Modification sites and domains are represented with different color. Abbreviations: P (phosphorylation site); S (serine); K (lysine); T (threonine); Y (tyrosine). (Data obtained from PhosphoSitePlus).
In pancreatic acini, the activation of soluble AC with HCO3- enhances secretagogue-stimulated cyclic AMP levels and inhibits secretagogue-stimulated zymogen activation and cell vacuolization (84).
C. Intracellular Targets of cyclic AMP
All the protein targets described below have a cyclic nucleotide-binding domain (CNBD) that has been conserved across a wide range of proteins, including the bacterial transcription factor catabolite activator protein (CAP) (166).
Protein kinase A
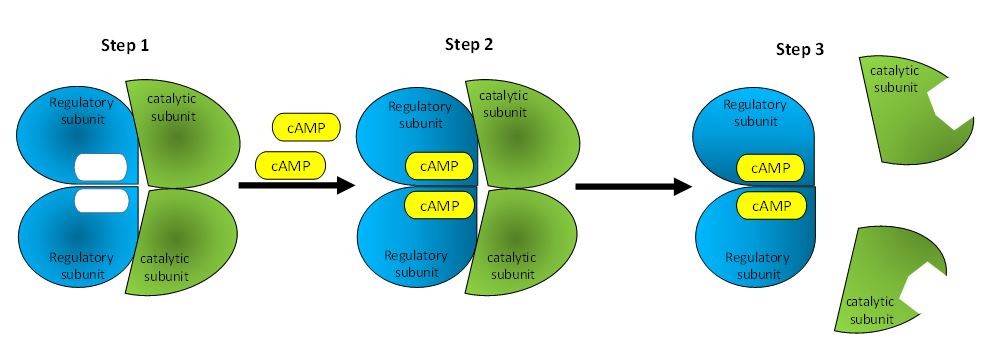
Cyclic AMP stimulates protein kinase A (PKA), which phosphorylates a number of cellular proteins by transferring a phosphate from ATP to a serine or a threonine located in sequence of residue of target protein. PKA contains two regulatory subunits, which possess the cyclic nucleotide binding domain (CNBD), and two catalytic subunits, which are responsible for the Ser/Thr phosphorylation. Upon binding of cyclic AMP to the two regulatory subunits, the two catalytic subunits are detached from the regulatory subunits and become active (161). The steps implicated in the activation of PKA by cyclic AMP are described in Figure 6.
Figure 6. Schematic representation of the activation of protein kinase A (PKA) by cyclic AMP. PKA is composed of four subunits: two regulatory and two catalytic. In the absence of cyclic AMP, the regulatory subunit inhibits the catalytic subunit. Upon binding of cyclic AMP to regulatory subunits, the regulatory subunits change conformation. The catalytic subunits become detached and able to phosphorylate target proteins in the cell.
The presence of PKA in pancreas was first reported in acinar cells from guinea pig (73). PKA catalyzes the phosphorylation of regulatory proteins associated with the pancreatic exocytotic process (17, 18). However, PKA does not appear to directly participate in pancreatic amylase secretion because the inhibitor of PKA, H-89, does not modify either basal or cyclic AMP-dependent secretagogues- stimulated amylase secretion from mouse pancreatic acini (132). Unlike in mouse acinar cells, in sealed mouse ducts PKA plays an essential role in the regulation of fluid secretion (133).
One of the important targets of PKA is the transcription factor cyclic AMP response element binding protein (CREB). Similar to other cell types, in pancreatic acini CREB phosphorylation at Ser133 increases upon PKA activation (132). The phosphorylation of CREB promotes the formation of a transcriptional complex on the cyclic AMP (cyclic AMP) response element (CRE) of certain promoters. The complex contains three proteins: 1) CREB, 2) the CREB-binding protein (CBP) and 3) CREB-regulated transcription coactivator 2. Its role is to stimulate the gene expression of certain proteins implicated in the regulation of metabolism, signaling, proliferation, differentiation, survival and oncogenesis.
Other important targets of PKA are the cystic fibrosis transmembrane conductance regulator (CFTR), 1,-4,-5-inositol trisphosphate receptor (IP3R), A-kinase anchoring proteins (AKAPs), ERK 1/2, and some isoforms of phosphodiesterase (PDE).
In pancreatic duct cells, PKA phosphorylates CFTR located in the apical membrane, on its regulatory domain which then enables channel gating (opening and closing) and Cl- secretion (5). Cyclic AMP evokes Cl- currents through CFTR, which mediates fluid transport across the luminal surfaces of pancreatic epithelial cells (22). In pancreatic acinar cells, PKA phosphorylates only one of the three IP3R isoforms, IP3R-3 (94, 155). The phosphorylation of IP3R-3 by PKA causes IP3-induced Ca2+ release, which is decreased in terms of the magnitude and kinetics of Ca2+ release (51, 155). Another important target of PKA are the A-kinase anchor proteins (AKAPs), which are a family of structurally related proteins consisting of more than 50 members (19). AKAP-150 has been shown to play a relevant role in the regulation of Na+/K+ ATPase pump activity in the homologous parotid gland (93, 140). Cyclic AMP also phosphorylates and thereby increases the activity of phosphodiesterases PDE3, PDE4, and PDE5 through PKA-induced phosphorylation (30, 184). Both PDE3 and PDE4 are cyclic AMP-specific PDEs, whereas PDE5 is a cyclic GMP-specific PDE (30).
Exchange protein directly activated by cyclic AMP (Epac)
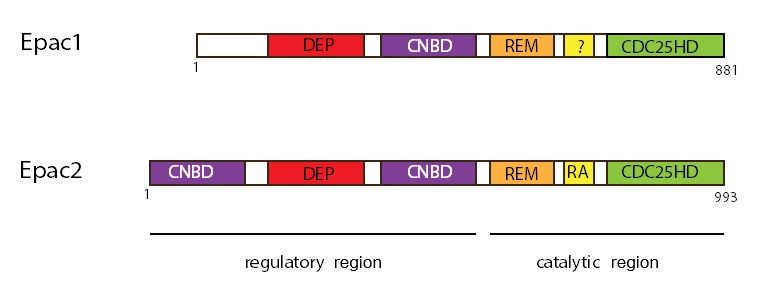
Cyclic AMP stimulates Epac (35). There are two isoforms of Epac: Epac1 and Epac2 (162). Both isoforms are homologous proteins with Epac2 having an N-terminal extension. They share common domain structures within a N-terminal regulatory region and a C-terminal catalytic domain (Figure 7)(12, 52, 65). The N-terminal regulatory region possesses one (Epac1) or two (Epac2) cyclic nucleotide-binding domains (CNBD) and a DEP (Dishevelled, Egl-10, and Pleckstrin) domain responsible for its localization to the plasma membrane. The C-terminal region contains CDC25-homology domain, a REM (Ras exchange motif) domain required for stabilizing GEF activity, and the GEF domain, which exerts GEF activity toward the small G proteins Rap1 and Rap2 (25). Epac1 is found in both pancreatic acini and ducts (23, 132, 133) and participates in cyclic AMP-stimulated amylase secretion (23, 132). Epac actives Rap1 (35), which is a small G-protein localized on zymogen granules as shown by both mass spectrometry and immunocytochemistry (26) and implicated in pancreatic amylase secretion (132). In addition to its role in pancreatic amylase secretion, Epac regulates exocytosis in pancreatic beta cells. Incretin-induced insulin secretion is mediated by Epac2, the primary isoform of Epac in pancreatic beta cells (75, 120, 146).
Figure 7. Domain structure of Epac1 and Epac2. Both Epac1 (881 amino acids) and Epac2 (993 amino acids) contain two regions: regulatory and catalytic regions. The regulatory region has the cyclic nucleotide-binding domain (CNB), and the Desheveled-Egl-10-Pleckstrin (DEP), which is responsible for the membrane localization. The catalytic region has the CDC25-homology domain, which is responsible for the guanine-nucleotide exchange activity; the Ras exchange motif (REM), which stabilizes the catalytic helix of CDC25-HD; and the Ras-association (RA) domain, which is a protein interaction motif.
Cyclic nucleotide-gated channels (CNG)
CNG channels are nonselective tetrameric cation channels that mediate Ca2+ and sodium influx in response to direct binding of intracellular cyclic nucleotides (7, 10). The mammalian CNG channel genes fall into two different gene families. One of these subfamily consists of four members CNGA1, CNGA2, CNGA3 and CNGA4, which represent the principal subunits that, except for CNGA4, form functional channels (77). The core structural unit consists of six transmembrane segments, designated S1-S6, cyclic nucleotide-binding domains (CNBD) near the C-terminal region. A pore region of ~20-30 amino acids is located between S5 and S6. The S4 segment in CNG channels resembles the voltage-sensor motif found in the S4 segment of voltage-gated K+, Na+, and Ca2+ channels. Both N-terminal and C-terminal regions are located in the cytoplasmic side and a glycosylated segment connecting S5 to the pore region is extracellular (77). The functional role of CNGs is well-studied in retinal rod photoreceptors (105), sperm (167) central nervous system (39) and cardiac excitability (61). Studies of CNG channels in exocrine tissues have not been reported.
CNG channels belong to a heterogeneous gene superfamily of pore-loop cation channels that share a common transmembrane topology and pore structure. Other members of this superfamily are the hyperpolarization-activated cyclic nucleotide-gated channel (HCN) (11), the ether-a-gogo (EAG) and human eag-related gene (HERG) family of voltage-activated K+ channels (44). HCN channels are principally operated by voltage and permeable to both Na+ and K+. Opening of HCN channels causes hyperpolarization of the membrane. Unlike CNG, in which cyclic nucleotides are strictly required to open the channel in HCN, cyclic nucleotides facilitate the opening by shifting the voltage dependence of activation to more positive values (11). Cyclic AMP has shown to modulate HCN channel activity through a PKA-dependent mechanism (13, 21).
The basolateral voltage-activated K+ channels, which belong to the HCN channel subfamily, are necessary for the regulation of Cl- secretion from pancreatic acini. In the rat pancreatic acinar cells, the presence of K+ channels in the basolateral membrane causes a membrane hyperpolarization, which provides the driving force for Cl- efflux. In addition, the efflux of K+ balances the K+ uptake by the Na+, K+ ATPase pump and other co-transporters (79). The functional and pharmacological properties of these channels are conferred once KCNE1 co-assembles with KCNQ1 (165). Both KCNE1 and KCNQ1 genes are expressed in rodent pancreas (159, 180). Cyclic AMP (79, 95) and carbachol (78) increase the amplitude of the slowly activating voltage-dependent K+ channel current (IKs) in rat pancreatic acinar cells.
RNA-binding protein
The Ca2+-regulated heat-stable protein of 24 kDa (CRHSP-24, also known as CARHSP1) is a serine phosphoprotein originally identified as a physiological substrate for the Ca2+-calmodulin regulated protein phosphatase calcineurin (PP2B) (54). In pancreatic acini, cyclic AMP partially dephosphorylated CRHSP-24 on at least two sites (141) through the activation of a phosphatase inhibited by calyculin A and okadaic acid, namely a PP2A or PP4 (141).
D. Regulation of the Adenylyl Cyclase/Cyclic AMP Signaling
The cytosolic levels of cyclic AMP are modulated by regulating GPCR activity, G protein activity, adenylyl cyclase activity, and cyclic AMP degradation.
Receptor regulation
GPCRs can be regulated in several ways. One way is through phosphorylation of specific amino acids in their cytosolic domain. When these amino acids are phosphorylated, the receptor becomes desensitized. G-protein-coupled receptor kinase (GRKs) are proteins that specifically phosphorylate GPCRs. Two GRKs have been found in the pancreas: GRK5 (92) and GRK6 (8). PKA can also phosphorylate GPCRs. In mouse pancreatic acini, VPAC receptors appear to be regulated by PKA phosphorylation based on the inhibition of PKA activity using a PKA inhibitor (H-89) causing up to two-fold increase in VIP-stimulated cyclic AMP formation (133).
G-protein regulation
G-protein activity can be affected by various toxins, with the two best studied being cholera toxin and pertussis toxin. Cholera toxin in complex with NAD+ and GTP-bound ADP-ribosylation factor 6 (ARF6-GTP) catalyzes the ADP-ribosylation of the α-subunit of Gs protein and prevents it from hydrolyzing its bound GTP, thereby locking the Gs protein in the active state, which causes the continuously activation of transmembrane AC (74). In guinea pig pancreatic acini cholera toxin increases cyclic AMP levels and amylase secretion (49). In rodent pancreatic acini, cholera toxin increases amylase secretion (34, 150). Its effect is potentiated by cholecystokinin and is less marked than in guinea-pig pancreatic acini (150). Unlike cholera toxin, pertussis toxin modifies the α-subunit of Gi protein and locks the Gi protein in the inactive state. The toxin catalyzes the ADP-ribosylation of a cysteine residue at position-4 from the C-terminal of the α-subunit of Gi protein, inhibiting the interaction of this protein with the receptor and attenuating the intracellular transduction (1, 45, 86). In rabbit pancreatic acini, pertussis toxin enhances CCK-induced cyclic AMP levels without affecting cholecystokinin (CCK)-induced Ca2+ mobilization or amylase secretion (168). In rat pancreatic acinar cells, although the pretreatment with either pertussis toxin or cholera toxin does not modify CCK-stimulated intracellular Ca2+ levels or phosphoinositide hydrolysis (107), pertussis toxin increases the basal levels of cyclic AMP and amylase secretion (156). Regulators of G-proteins signaling (RGS) molecules, which catalyze the GTP hydrolysis of heterotrimeric G-proteins, play a critical role in regulating G-protein activity. RGS1, RGS2, RGS4, RGS16 and GAIP, have been found in isolated pancreatic acinar cells using RT-PCR (103). Although their function in the regulation of Gs activity in exocrine pancreas is still unknown, in olfactory neurons RGS2 decreases Gs-stimulated cyclic AMP levels (152).
Adenylyl Cyclase regulation
Adenylyl cyclase activity can be regulated by distinct intracellular signals. As previously indicated in Table 1, transmembrane ACs are classified into four groups: Group I consists of Ca2+ -stimulated (AC1, AC3, AC8); Group II consists of Gβγ-stimulated (AC2, AC4, AC7); Group III consists of Giα/Ca2+/PKC/PKA-inhibited (AC5, AC6); Group IV consists of Ca2+-inhibited (AC9), which is forskolin-insensitive (134, 138, 170). Recently, AC9 activity has also been shown to be inhibited by Gαi/o proteins and PKC (33). The Ca2+-binding protein involved in the stimulatory effect of Ca2+ on the group I is calmodulin, which forms an active Ca2+-calmodulin complex. Calmodulin is present in pancreatic acini and activated by CCK (42). The Ca2+-calmodulin complex binds to the calmodulin-binding site present in the Group I isoform and increases its activity dramatically. AC9 is also stimulated by calmodulin (32). The Ca2+-binding protein involved in the inhibitory effect of Ca2+ on AC9 is calcineurin, which is a serine/threonine protein phosphatase activated by CCK (54, 56) and involved in amylase secretion from rat pancreatic acini (53), as well as caerulein-induced intracellular pancreatic zymogen activation (67).
Forskolin is a diterpene extracted from the root of the plant Coleus forskohlii that directly activates all transmembrane AC isoforms, except AC9 (128, 144) by interacting with the two cytoplasmic domains (C1 and C2), that form the catalytic domain (157). The lack of effect of forskolin on AC9 may be accounted for by the residues Tyr1082 and Ala1112 (177).
Unlike transmembrane AC, AC10 is not activated by either G protein or forskolin. Its activation is dependent on the HCO3- levels (27), though it can also be activated by divalent cations, such as Ca2+, Mg2+ and Mn2+ (99). A combination of Ca2+ and HCO3- activates soluble adenylyl cyclase synergistically (99). AC10 is also activated by changes in intracellular pH (117).
The most common post-translational modification of an AC isoform is the phosphorylation of a serine, threonine or tyrosine residue (Figures 3 and 4). Phosphorylation of AC1 and AC3 by Ca2+/calmodulin kinases inhibits the cyclase activity by blocking the binding site. Phosphorylation of ACs by either PKA or PKC causes an inhibition of the enzyme activity. Ubiquitination and acetylation are other modifications found in the human AC isoforms, though their consequences in AC activity are still unknown.
Regulation of cyclic AMP degradation
Cyclic AMP degradation is carried out by the enzyme phosphodiesterase (PDE), which is an exonuclease capable of hydrolyzing a phosphate ester and pyrophosphate bonds, and thereby, converting cyclic AMP into 5’AMP (30). Eleven PDE isoforms exist and each has unique biochemical properties. PDE1, which hydrolyzes both cyclic AMP and cyclic GMP, has been found in pancreatic acini using immunocytochemistry (112), whereas PDE7B, a cyclic AMP-specific PDE, has been found in whole pancreas using Northern blotting (62). PDE4, which is highly expressed in most immune and inflammatory cells and a cyclic AMP-specific PDE, is involved in the development of acute pancreatitis because the selective inhibitor rolipram attenuates the severity of acute pancreatitis in rats (110).
E. Role for Adenylyl cyclase/Cyclase AMP pathway in Pancreatic Exocrine Cells
The exocrine pancreas is primarily composed of pancreatic acini and ducts. Pancreatic acini synthesize and release digestive enzymes into the duodenum, whereas pancreatic ducts release a HCO3--rich fluid to neutralize the acidic chyme released from the stomach. In this section the roles for adenylyl cyclase/cyclic AMP pathway are described.
Pancreatic duct HCO3--rich fluid
Secretagogues, such as secretin and vasoactive intestinal polypeptide (VIP), increase cyclic AMP and stimulate HCO3--rich fluid secretion from pancreatic duct cells (69, 70, 126). An increase in the levels of cyclic AMP, through PKA phosphorylation, activates CFTR to recirculate chloride back into the glandular lumen, and thereby, depolarizes both luminal and basolateral membranes. Depolarization of the basolateral membrane increases the driving force of an electrogenic sodium-HCO3- co-transporter on the basolateral membrane leading to the entry of HCO3-, which is then secreted at the apical membrane via the Cl-/HCO3- exchanger (5). The AC6/cyclic AMP/PKA pathway has an important role in the physiological function of pancreatic ducts because the VIP-stimulated expansion of the lumen observed in vitro in pancreatic ducts from WT mice upon VIP stimulation was absent in duct fragments from AC6-deficient mice. In vivo collection of pancreatic fluid also showed a decrease in fluid secretion from AC6-deficient mice (133). The secretory effect is highly dependent on PKA activation because in isolated pancreatic ducts from AC6-deficient mice PKA activation was abolished in response to VIP, secretin, and forskolin (133, 134).
Several ion channels are affected by cyclic AMP/PKA pathway. PKA phosphorylates CFTR located in the apical membrane of the pancreatic duct cells (5). Elevation of intracellular cyclic AMP by stimulation with forskolin significantly inhibits the Na+/H+ exchanger (NHE) and this, like the stimulation of the apical anion exchanger, may occur through a direct physical interaction with CFTR (5). The basolateral Cl-/HCO3- exchanger (AE) does not seem to be directly activated by forskolin (96). For more details, see Chapter “Molecular Mechanisms of Pancreatic HCO3- Secretion”.
Pancreatic acini enzymatic-rich fluid
Early work showed that a number of compounds that increase cyclic AMP levels stimulate amylase secretion from pancreatic acini (2, 16, 29, 50, 109, 118, 119, 156). Phosphodiesterase inhibitors, such as 3-isobutyl-1-methylxantine, increase pancreatic amylase secretion (48). Pertussis toxin catalyzes the ADP-ribosylation of a cysteine residue at postion-4 from the carboxyl-terminal domain of the α-subunit of Gi protein, inhibiting the interaction of this protein with the receptor and impairing intracellular transduction. Treatment with pertussis toxin causes an increase in cyclic AMP levels and amylase secretion from rat pancreatic acini (168), where multiple pertussis toxin-sensitive G proteins have been found (e.g. Gi1, Gi2, Gi3 and Go) (142). Forskolin interacts with the two cytosolic domains C1 and C2 of transmembrane ACs, except AC9 (138). Forskolin slightly stimulates amylase secretion in rat (37, 80) and potentiates the response to Ca2+-dependent secretatogues (59). Recently, pancreatic acini from AC6 deficient-mice showed a reduction in stimulated amylase secretion and PKA activity (133). Because this inhibition was only partial, it is likely that other AC isoforms expressed in pancreatic acini and/or Epac1, which has participated in cyclic AMP-stimulated amylase secretion (23, 132) are also responsible for the secretory role of pancreatic acini. The result showing the deletion of AC6 does not affect the response to the Epac1 analog 8-pCPT-2’-O-Me-cyclic AMP on amylase secretion supports this hypothesis (133).
Glucagon-like peptide-1 (GLP-1) is a glucoincretin hormone secreted by intestinal L cells in response to nutrient ingestion that can act through its receptor (GLP-1R) (41). GLP-1R is expressed in isolated mouse pancreatic acini and mediates GLP-1-induced amylase secretion from isolated mouse pancreatic acini through cyclic AMP/PKA pathway activation (66).
Both VIP and secretin, through the phosphorylation of p21-activated kinase (PAK) 4, but not PAK2, cause an activation of the Na+/K+ ATPase in isolated rat pancreatic acini and, as a consequence, an increase in pancreatic acinar fluid secretion; Epac mediates VIP-induced PAK4 activation, whereas PKA mediates secretin-induced PAK4 activation (130).
Differentiation, transdifferentiation and proliferation
Cyclic AMP plays an important role in differentiation, transdifferentiation and proliferation of pancreatic cells. Isolated adult islets of Langerhans were able to transdifferentiate to duct epithelial-like cyst structures in presence of elevated cyclic AMP and a solid extracellular matrix (e.g. matrigel and collagen I) (164). The presence of intracellular cyclic AMP elevating factor, such cholera toxin, was also required for the proliferation and maintenance of pancreatic epithelial duct cells (176). However, transforming growth factor-β (TGF-β), which is an important regulator of growth and differentiation in the pancreas, can activate PKA without affecting cyclic AMP levels in pancreatic acini (179). TGF-β-mediated growth inhibition and TGF-β -induced p21 and SnoN expression are mediated by PKA because both effects were blocked the PKA inhibitors H89 and PKI peptide (179). A physical interaction between a Smad3/Smad4 complex and the regulatory subunits of PKA has been shown in pancreatic acini (179).
Development of Pancreatitis
Acute pancreatitis is an acute inflammatory disease of the pancreas. The disease appears to be initiated when a pathologic factor like alcohol or bile injuries the acinar cell and it responds by releasing inflammatory mediators and by activating digestive enzymes, especially proteinases, and restricting their secretion. These events initiate a cascade that leads to pancreatic inflammation and local and systemic tissue injury (175). The participation of cyclic AMP in the development of pancreatitis has been studied by the Gorelick lab. An early study showed that cyclic AMP-dependent secretatogues sensitizes the pancreatic acinar cells to zymogen activation induced by caerulein, a CCK analog (101). The same research group in a subsequent work showed that cyclic AMP, by enhancing the release of pancreatic enzymes from the acinar cell, can overcome the acinar cell injury induced by high concentrations of carbachol, a cholinergic agonist (24). Recently, the inhibition of soluble AC by KH7 was shown to enhance the activation evoked by caerulein of two important digestive enzymes chymotrypsinogen and trypsinogen, as well as caerulein-stimulated amylase secretion from rat pancreatic acini (84). Together these studies suggest a complex role for cyclic AMP in acute pancreatitis in which it may enhance some pancreatitis responses while simultaneously lessening the effects of others.
F. Role for Adenylyl cyclase/Cyclase AMP pathway in Pancreatic Cancer
Of all the different cancers, pancreatic cancer is one of the major unsolved health problems. In 2020, the US projected estimates report that the number of deaths from pancreatic cancer will be over 47,050 (148). This is a 2.8 % increase in estimated deaths from 2019 (147). Unfortunately, the usual cancer treatment options do not have much of a positive effect. For that, it is of interest to develop new clinical strategies to increase the survival of patients with pancreatic cancer.
In 1996, receptors for VIP and β-adrenergic agonists (i.e., adrenaline and isoproterenol), which are functionally coupled to AC, were found in five human pancreatic adenocarcinoma cell lines BxPC-3, Hs 766T, Capan-2, Panc-1, and Capan-1 (Hs 766T and BxPC-3 were the most responsive, followed by Capan-2 and Capan-1, and finally Panc-1; MIA PaCa-2 cells did not respond to any of the agonists tested) (3). Because high concentration of secretin was necessary to stimulate AC, the process of neoplastic transformation has either downregulated the expression of secretin receptors or led to a defect in the receptor itself (3).
Later, the Gαs/AC/cyclic AMP pathway was shown to participate in the development of pancreatic pre-neoplastic lesions and the regulation of multiple pancreatic cancer cellular processes, including migration and invasion (15, 185, 187). Support for the AC/cyclic AMP pathway in developing pancreatic pre-neoplastic lesions has come from a study showing the presence of a mutation in the oncogene GNAS, which results in constitutive activation of Gαs (87),in two precursor lesions of pancreatic adenocarcinoma: the pancreatic intraepithelial neoplasia (PanIN) and the macroscopic intraductal papillary mucinous neoplasm (IPMN) (173). Two different groups have shown that the co-expression of GNASR201C and KRasG12Dpromote murine pancreatic tumorigenesis, mimicking the pre-neoplastic lesions PanIN and IPMN (68, 158). However, GNASR201C does not seem to cooperate with the oncogene KRasG12D as other mutants do (e.g., TP53, SMAD4 and CDKN2A). GNASR201C functions as an “onco-modulator” that affects the phenotype of pre-neoplastic lesions arising in the context of mutant KRASG12D and leads to invasive carcinomas with a predominantly well-differentiated morphology (68). The early activation of hippo pathway by Gαs/cyclic AMP/PKA is likely to be the downstream pathway responsible for the differentiation in GNASR201C and KRasG12Ddouble positive cells because the co-expression of these mutants causes phosphorylation of the transcriptional co-activator YAP1, which is sequestered and inactivated in the cytoplasm, by Hippo kinase cascade (68).
Support for the AC/cyclic AMP pathway in inhibiting migration and invasion has come from a number of studies using forskolin as a stimulator of AC (15, 129, 187). From all the transmembrane isoforms present, AC3 was more highly expressed in the pancreatic tumor tissue compared to the adjacent non-tumor tissues and in two pancreatic cancer cell lines, HPAC and PANC-1, compared to the normal pancreatic ductal cell line (hPDEC). AC3 was also the isoform responsible for the inhibitory effect of forskolin on cell migration and invasion in pancreatic cancer cell lines HPAC and PANC-1 (129). The mechanism by which forskolin/AC3/cyclic AMP pathway inhibits cell migration and invasion involves the quick formation of AC3/CAP1 complex and sequestration of G-actin, which leads to an inhibition of filopodia formation and cell motility (129).
The stimulation of the AC/PKA pathway by forskolin and VIP increases expression and release of apomucin MUC5AC from pancreatic cancer cells SW1990 (63). Because the co-expression of mutant GNASR201C and KRasG12Dup-regulates apomucins MUC1 and MUC5AC, but not MUC2, which resembles the expression of apomucins in human IPMNs (68), GNASR201C mutation, by Gαs/cyclic AMP/PKA pathway, might induce the expression of MUC5AC in IPMNs.
IV. Guanylyl Cyclase/Cyclic GMP Signaling
Cyclic GMP is made from GTP through a catalytic reaction mediated by guanylyl cyclase (GC). Like AC, GC can be transmembrane or soluble. Unlike transmembrane ACs, all transmembrane GCs share a basic topology, which consists of an extracellular ligand binding domain, a single transmembrane region and an intracellular domain that contains a juxtamembranous protein kinase-homology domain (KHD), a coiled-coil amphipathic α-helical or hinge region, and the catalytic GC domain at its C-terminal end. The function of the KHD is still unknown. Although it binds ATP and contains several residues conserved in the catalytic domain of protein kinases, kinase activity has not been found. In fact, it regulates the GC activity at the C-terminal end. The coiled-coil hinge region is involved in the process of dimerization, which is essential for the activation of GC domain (89). There are at least seven transmembrane guanylyl cyclases: GC-A, GC-B, GC-C, GC-D, GC-E, GC-F, and GC-G (Figures 8 and 9). Only GC-A, GC-B and GC-C have shown to regulate the function of exocrine pancreas.
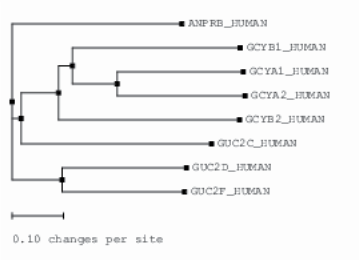
Figure 8. Phylogenetic tree of the transmembrane and soluble GC sequence. A dendrogram shows the degree of relatedness of sequence of GC isoforms built by ClustalW. The length of each horizontal line indicates estimated evolutionary distance. Branches separate an individual subfamily. The order of evolution as followed (from the most conserved to the less conserved): ANP RB (also known as NPR-B or GC-B);GUC2C (GC-C); GUC2D (GC-D, retinal);GCYB2 GC (GC soluble subunit β2); GCYB1; GCYA1 (GC soluble subunit α1). GUC2D and GUC2F, as well as GCYA1 and GCYA2, are co-evolutionary isoforms.
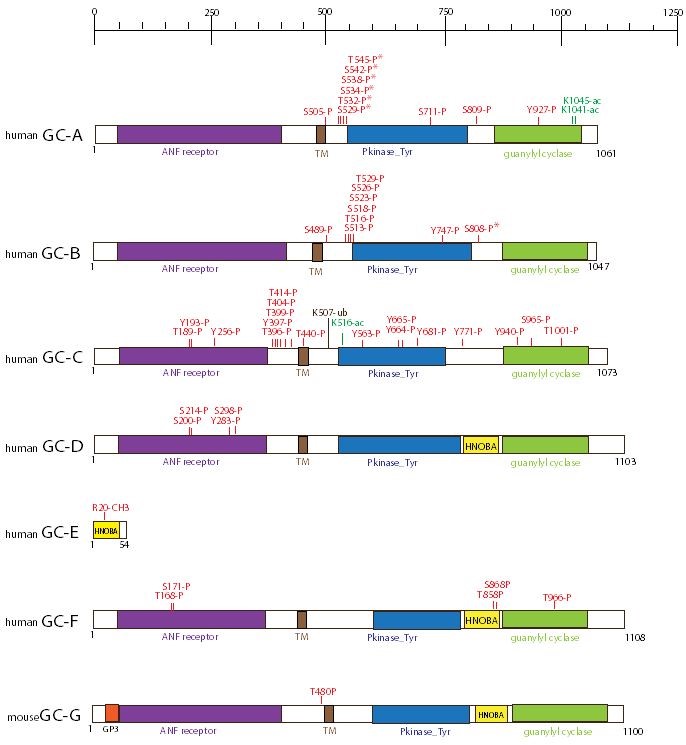
Figure 9. Schematic representation of the domain structure of the seven GC isoforms. The number of amino acid residues is reported on the side of each structure. Modifications sites and domains are represented with different color. The transmembrane GC isoforms share a common structure consisting of an extracellular ligand binding domain, a short transmembrane region (TM), and an intracellular domain that contains the catalytic region (GC) at its C-terminal end. Abbreviations: TM (transmembrane segments); ac (acetylation); P (phosphorylation site); ub (ubiquitination); CH3- (methylation); S (serine); K (lysine); R (arginine); T (threonine); Y (tyrosine). * (sites implicated in the activity of the enzyme). (Data obtained from PhosphoSitePlus).
A. Transmembrane GC
There are at least two groups of ligands for transmembrane GC; natriuretic peptides, and guanylin and uroguanylin peptides.
Natriuretic peptides
There are three members of natriuretic peptide family: atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (Figure 10A). The actions of natriuretic peptides are mediated by the activation of three transmembrane receptor subtypes: natriuretic peptide receptor type A (NPR-A, also known as GC-A), type B (NPR-B, also known as GC-B) (Figure 10B), and type C (NPR-C). The last one (NPR-C) is not a transmembrane GC; it was initially considered to be a clearance receptor whose only function was to regulate the plasma concentration of natriuretic peptides (106). Later, NPR-C receptors have been found to be coupled to the inhibition of AC through α subunit of an inhibitory guanine nucleotide regulatory protein (Gαi1/Gαi2) and/or the activation of phospholipase C (PLC) through the βɣ subunits of the same Gi protein (104, 115, 121, 122) in a number of tissues, including pancreatic acini (135). Because NPR-C receptors are not coupled to cGMP generation, NPR-C receptors are not further discussed in this chapter.
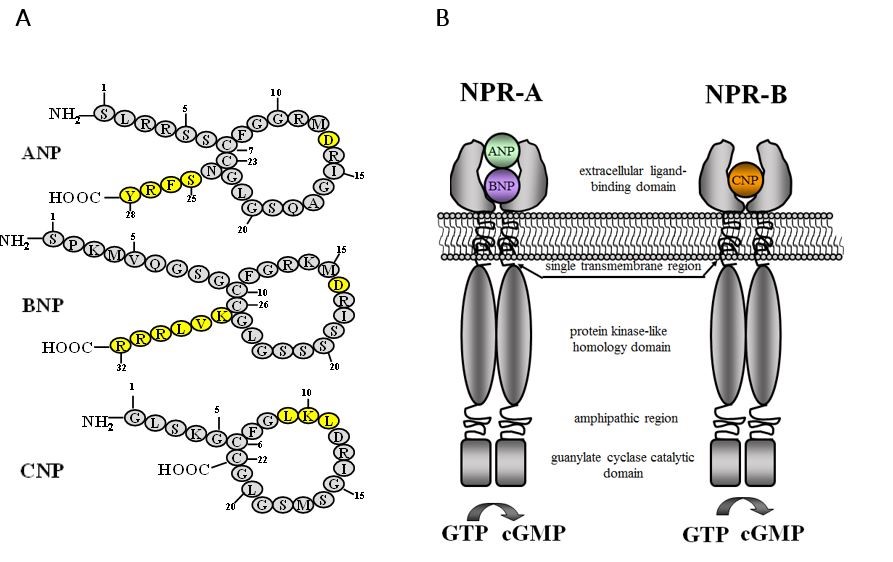
Figure 10. (A) Schematic representation of the amino acid sequence and the structure of biological active natriuretic peptides ANP, BNP and CNP. The members of the natriuretic peptide family share a common structure, which consists of a 17-amino acid bonded loop bridge by intracellular disulfide bond required for the natriuretic and diuretic activity. Note the amino acids with a yellow color are also important for their activity. (B) Schematic representation of natriuretic peptides receptors NPR-A and NPR-B. The structure of NPR-A and NPR-B receptors possesses three domains: the extracellular ligand-binding domain, the intracellular protein kinase-like homology domain, and GC catalytic domain.
The NPR-A and NPR-B receptors, whose relative molecular mass is 130-180 kDa, have a similar structure that contains four domains: an extracellular ligand-binding domain, a single transmembrane domain, an intracellular tyrosine-like domain, an amphipathic region and a GC catalytic domain. Upon ligand binding, the NPR-A and NPR-B receptors change their conformation which results in GC activation and cyclic GMP generation (123, 131).
Guanylin and uroguanylin
Guanylin and uroguanylin are peptides secreted from the intestine, which influence electrolyte and fluid transport in the intestine and kidney, respectively (46, 149). Their effects are mediated by the NPR-C receptor, which is predominately expressed in the intestine.
Soluble GC
Soluble GC is a histidine-ligated hemoprotein that consists of two homologous subunits, α and β. The well-known isoform is the α1β1 protein; α2β2 subunits have also been identified (57, 182). Each soluble GC subunit consists of four domains: an N-terminal heme-Nitric Oxide Oxygen (H-NOX) domain (also called a SONO domain); a central Per-ARNT-Sim (PAS) domain; a coiled-coil domain; and a C-terminal catalytic cyclase domain. The β1 subunit contains a N-terminal heme-binding domain, a Per/Arnt/Sim (PAS) domain, a coiled-coil domain, and a C-terminal catalytic domain (20), as described in Figure 11.
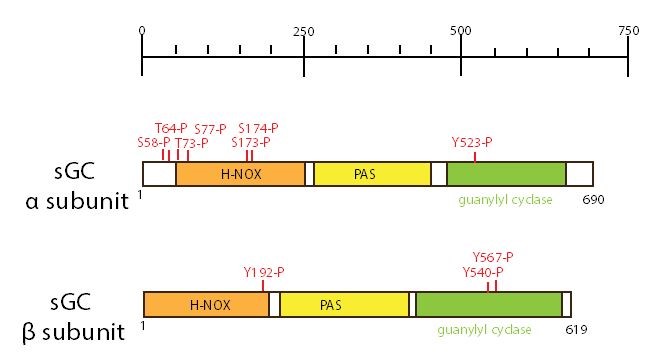
Figure 11. Schematic representation of the domain structure of soluble GC (α subunit and β subunit) is represented. The number of amino acid residues is reported on the side of each structure. Modifications sites and domains are represented with different color. Each soluble GC subunit consists of four domains: a N-terminal Heme-Nitric Oxide Oxygen (H-NOX) domain (also called a SONO domain); a central Per-ARNT-Sim (PAS) domain; a coiled-coil domain; and a C-terminal catalytic cyclase domain. Abbreviations: P (phosphorylation site); S (serine); T (threonine); Y (tyrosine). (Data obtained from PhosphoSitePlus).
The PAS domain mediates protein-protein interactions and have often been found to bind heme, a flavin, or a nucleotide (111). The coiled-coil domain, appears to be unique to soluble GC (38). The functions of PAS and coiled-coil domains are still unknown. The catalytic domain is localized at the C-terminal 467-690 and 414-619 residues of the α1 and β1 subunits, respectively (171). The catalytic domains must form a heterodimer for cyclic GMP synthesis, and in the full length protein (38). The C-terminal regions of the α1 and β1 subunits are highly homologous to the particulate GC and AC catalytic domains (38). Soluble GC binds nitric oxide (NO), which is its primary activator (102), and can also be activated by carbon monoxide, but not oxygen (38). NO is a gaseous second messenger molecule synthesized from L-arginine and oxygen by the enzyme NO synthase. NO binds to the heme cofactor of soluble GC. The binding of NO to soluble GC leads to an increase in cyclic GMP.
B. Intracellular Targets of cyclic GMP
Intracellular targets of cyclic GMP, like intracellular targets of cyclic AMP, have a cyclic nucleotide-binding domain (CNBD) in their structure.
Cyclic nucleotide-gated channels
Cyclic nucleotide-gated channels (CNG) have been described above (see2.2.Intracellular Targets of cyclic AMP). The physiological significance of cyclic GMP as activating agent of CNG has been described in photoreceptor cells and olfactory sensory neurons, where CNG play an important role in sensory transduction (10). There are no reports of CNG function in an exocrine tissue.
Cyclic GMP-dependent protein kinase
The increase in the levels of cyclic GMP activates cyclic GMP-dependent serine/threonine protein kinase (PKG). Two genes prkg1 and prkg2 code for the two isoforms PKGI and PKGII (64). The human prkg1 gene is located on chromosome 10 at p11.2 –q11.2 and has 15 exons. The N-terminus of PKGI is encoded by two alternative exons that produce the isoforms PKGIα and PKGIβ. The human prkg2 gene is located on chromosome 4 at q13.1q21.1 and has 19 exons. Its transcript yields a protein with an apparent mass of 87.4 kDa (64). Like PKA, PKG is composed of two functional domains: a regulatory domain and a catalytic domain. The regulatory domain is subdivided into the N-terminal domain and the cyclic nucleotide-binding domain (CNBD) containing the high and low cyclic GMP affinity binding pockets. The catalytic domain contains the Mg2+-ATP- and peptide-binding pockets. Upon binding of cyclic GMP to the two regulatory subunits, the two catalytic subunits are released from the regulatory subunits and become active (64). The substrates of this kinase are P240, P132 and phospholamban, though none of them is a specific PKG substrate (47, 97).The intracellular levels of cyclic GMP are regulated by PDE enzymes, which hydrolyze cyclic GMP into 5’GMP (47). In pancreatic acinar cells from guinea pig, the presence of PKG activity has been reported (73).
C. Role for Guanylyl Cyclase/Cyclic GMP Pathway in Pancreatic Exocrine Cells
The role of GC/cyclic GMP in the regulatory function of pancreatic exocrine cells is still controversial. One of the first papers published on isolated pancreatic lobules from guinea pig and rabbit showed that carbamylcholine (carbachol), pancreozymin (now known as CCK), and caerulein all increased the levels of cyclic GMP without modifying the levels of cyclic AMP. The authors concluded that cyclic GMP is the second messenger involved in the process of stimulus- secretion coupling in the acinar cells of exocrine pancreas (58). Later, Ca2+ was shown to be an important mediator of the stimulus-secretion coupling process (169). Moreover, increased intracellular levels of cyclic GMP has a little or no effect on the stimulus-secretion coupling in pancreatic acinar cells (169, 181). However, cyclic GMP has been involved in the Ca2+ entry across the cell membrane to replenish the intracellular Ca2+ stores (124, 125).
The function of NO, the ligand for soluble GC, has been studied in the exocrine pancreas. NO can increase endogenous cyclic GMP and rat pancreatic secretory activity (181). NO triggers an increase in intracellular Ca2+ levels via cyclic GMP and inositol trisphosphate in pancreatic acinar cells (113). NO is localized in intrapancreatic ganglionic cells and efferent nerve fibers (100) and implicated in the control of mesenteric circulation (108). NO inhibits pancreatic exocrine secretion in dogs (85), and rats (172). NO production regulates cyclic GMP formation and Ca2+ influx in rat and guinea pig isolated pancreatic acini (55). Blocking NO production by chemical inhibitors of NO synthase, NG-monomethyl-L-arginine or NG-nitro-L-arginine, abolished cyclic GMP formation induced by the cholinergic agonist carbachol in a dose-dependent manner (55). NO has shown to have a protective role in acute pancreatitis (40, 71, 72).
The functions of two ligands for transmembrane GCs, natriuretic peptides, and guanylin, have also been studied in exocrine pancreas:
- Natriuretic peptides: All of the three receptors of natriuretic peptides are expressed in pancreatic acini (135) and both ANP and CNP increase intracellular levels of cyclic GMP in isolated pancreatic acini (60, 135). However, the action of ANP and CNP on pancreatic secretion is not mediated by an increase in cyclic GMP. Indeed, ANP and CNP increase pancreatic fluid and protein output through the NPR-C receptor activation/Ca2+ release (135-137).
- Guanylin and uroguanylin: In rat pancreatic acini, guanylin increases cyclic GMP levels, elicits a small amount of amylase secretion and a small Ca2+ transient (181). Guanylin is localized specifically to the centroacinar cells and proximal duct cells and released luminally into the pancreatic ducts based on its presence in the pancreatic juice (90). Functional studies in two different human pancreatic duct cell lines revealed that guanylin is an intrinsic pancreatic regulator of Cl- current activation in pancreatic duct cells via cyclic GMP. Using whole-cell patch-clamp forskolin increased of Cl- conductance mediated by cyclic AMP, while guanylin increased Cl- conductance mediated by cyclic GMP, but not cyclic AMP (91).
The existence of both membrane and soluble GCs in pancreatic acini suggest that there are two distinct sources of cyclic GMP located in different compartments, which could have different effects in pancreatic acini.
D. Role for Guanylyl Gyclase/Cyclic GMP Pathway in Pancreatic Cancer
Guanylin, uroguanylin and GC-C are expressed at mRNA and protein levels in pancreatic cancer specimens and cancer cell lines and uroguanylin inhibits pancreatic cancer cell proliferation in a concentration-dependent manner (82).
The transmembrane cell surface receptor GC-C has been identified in 60-70 % of pancreatic cancer. An Anti-GC-C antibody–drug conjugate TAK-264 (formally known as MLN0264) in patients with advanced or metastatic pancreatic cancer (NCT02202785) showed low efficacy and, for that, no further clinical investigation was undertaken (4).
PKG1 is expressed in pancreatic cancer cells and its inhibitor, DT3, causes cytotoxicity through necrosis and inhibits proliferation and migration of pancreatic cancer cells (76).
V. Non-Canonical Cyclic Nucleotides
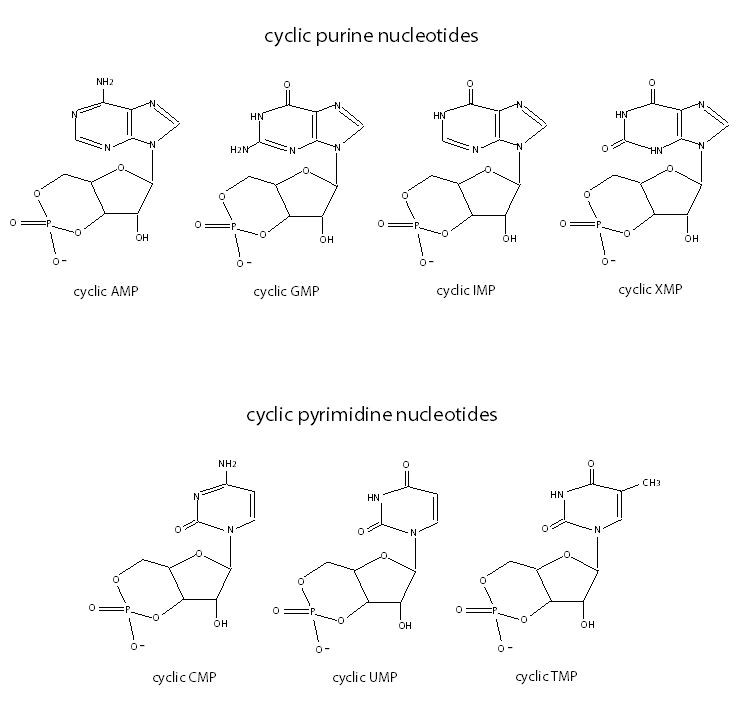
Cyclic IMP, cyclic XMP, cyclic CMP, cyclic UMP and cyclic TMP are cyclic nucleotides whose function is well-characterized (Figure 12). Using HPLC-MS/MS spectrometry, both cyclic CMP and cyclic UMP have been found in numerous cultured cell types and in human urine. Cyclic CMP and cyclic UMP concentrations are regulated by the cell proliferation status because growth-arrest of cells resulted in preferential decrease of cellular cyclic CMP and cyclic UMP concentrations over cyclic AMP and cyclic GMP concentrations. Previous findings suggest that cyclic CMP and cyclic UMP could play a role as second messengers because cyclic CMP and cyclic UMP-hydrolysing PDEs were found in mammalian tissues. Recently, soluble AC has shown to be responsible for the production of cyclic CMP and cyclic UMP in HEK293 and B103 cells because the soluble AC inhibitor KH7 decreased HCO3--stimulated cyclic nucleotide levels in concentration-dependent manner. Forskolin, which is a stimulator of all transmembrane ACs except AC9, does not affect the levels of cyclic CMP and cyclic UMP. The authors conclude that soluble AC may likely have a distinct role in the regulation of cyclic nucleotide levels compared to soluble GC, membrane GC and membrane AC (145). In RFL6 lung fibroblasts endogenously expressing soluble GC, NO-stimulated cyclic UMP formation were similar to cyclic GMP formation (6). In contrast to soluble GC, transmembrane GC do not induce cyclic UMP formation (9). Recently, cyclic CMP was found in several mouse tissues including pancreas as assessed by HPLC-MS/MS and HPLC-MS/TOF (143).
Figure 12. Schematic representation of cyclic purine (cyclic AMP, cyclic GMP, cyclic IMP, cyclic XMP) and pyrimidine (cyclic CMP, cyclic UMP and cyclic TMP) nucleotides.
Unlike cyclic CMP and cyclic UMP, cyclic TMP, cyclic IMP and cyclic XMP levels are very low to be detectable in cultured cell lines (9). Cyclic IMP levels increase in a hypoxic environment probably as a result of ATP deamination, which becomes ITP, and by soluble GC activity, ITP becomes cyclic IMP (145).
Non-canonical cyclic nucleotides have been studied so far in cardiovascular system, central nervous system and reproductive system. A description of their roles in the regulation of these system can be found in (46). To the best of our knowledge, at the present there is no data available for the role of non-canonical cyclic nucleotides in the digestive system.
VI. References
- Adamson PB, Hull SS, Jr., Vanoli E, De Ferrari GM, Wisler P, Foreman RD, Watanabe AM, and Schwartz PJ. Pertussis toxin-induced ADP ribosylation of inhibitor G proteins alters vagal control of heart rate in vivo. Am J Physiol 265: H734-740, 1993. PMID: 8368374.
- al-Nakkash L, Simmons NL, Lingard JM, and Argent BE. Adenylate cyclase activity in human pancreatic adenocarcinoma cell lines. Int J Pancreatol 19: 39-47, 1996. PMID: 8656026.
- Almhanna K, Wright D, Mercade TM, Van Laethem JL, Gracian AC, Guillen-Ponce C, Faris J, Lopez CM, Hubner RA, Bendell J, Bols A, Feliu J, Starling N, Enzinger P, Mahalingham D, Messersmith W, Yang H, Fasanmade A, Danaee H, and Kalebic T. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest New Drugs 35: 634-641, 2017. PMID: 28527133.
- Argent BE, Gray MA, Steward MC, and Case RM. Cell physiolgy of pancreatic ducts. In: Physiology of the Gastrointestinal Tract. 5th Ed. Vol 2. Edited by Johnson LR. London: Elsevier BV, 2012. p. 1399-1423. DOI: 10.1016/ B978-0-12-382026-6.00051-8.
- Bahre H, and Kaever V. Measurement of 2',3'-cyclic nucleotides by liquid chromatography-tandem mass spectrometry in cells. J Chromatogr B Analyt Technol Biomed Life Sci 964: 208-211, 2014. PMID: 242656940.
- Benarroch EE. HCN channels: function and clinical implications. Neurology 80: 304-310, 2013. PMID: 23319474.
- Benovic JL, and Gomez J. Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family. J Biol Chem 268: 19521-19527, 1993. PMID: 8366096.
- Beste KY, and Seifert R. cCMP, cUMP, cTMP, cIMP and cXMP as possible second messengers: development of a hypothesis based on studies with soluble guanylyl cyclase α1 β1. Biol Chem 394: 261-270, 2013. PMID: 23087103.
- Biel M, and Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol 111-136, 2009. PMID: 19089328.
- Biel M, Wahl-Schott C, Michalakis S, and Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847-885, 2009. PMID: 19584315.
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680-686, 2006. PMID: 17084085.
- Boulton S, Akimoto M, VanSchouwen B, Moleschi K, Selvaratnam R, Giri R, and Melacini G. Tapping the translation potential of cAMP signalling: molecular basis for selectivity in cAMP agonism and antagonism as revealed by NMR. Biochem Soc Trans 42: 302-307, 2014. PMID: 24646235.
- Buck J, Sinclair ML, Schapal L, Cann MJ, and Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A 96: 79-84, 1999. PMID: 9874775.
- Burdyga A, Conant A, Haynes L, Zhang J, Jalink K, Sutton R, Neoptolemos J, Costello E, and Tepikin A. cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: effects of PKA and EPAC. Biochim Biophys Acta 1833: 2664-2672, 2013. PMID: 23797058.
- Burnham DB, McChesney DJ, Thurston KC, and Williams JA. Interaction of cholecystokinin and vasoacive intestinal polypeptide on function of mouse pancreatic acini in vitro. J Physiol 349: 475-482, 1984. PMID: 6204039.
- Burnham DB, Sung CK, Munowitz P, and Williams JA. Regulation of protein phosphorylation in pancreatic acini by cyclic AMP-mediated secretagogues: interaction with carbamylcholine. Biochim Biophys Acta 969: 33-39, 1988. PMID: 2450590.
- Burnham DB, and Williams JA. Activation of protein kinase activity in pancreatic acini by calcium and cAMP. Am J Physiol 246: G500-508, 1984. PMID: 6326611.
- Calejo AI, and Tasken K. Targeting protein-protein interactions in complexes organized by A kinase anchoring proteins. Front Pharmacol 6: 192, 2015. PMID: 26441649.
- Cary SP, Winger JA, Derbyshire ER, and Marletta MA. Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci 31: 231-239, 2006. PMID: 16530415.
- Chang F, Cohen IS, DiFrancesco D, Rosen MR, and Tromba C. Effects of protein kinase inhibitors on canine Purkinje fibre pacemaker depolarization and the pacemaker current i(f). J Physiol 440: 367-384, 1991. PMID: 1804968.
- Chanson M, Scerri I, and Suter S. Defective regulation of gap junctional coupling in cystic fibrosis pancreatic duct cells. J Clin Invest 103: 1677-1684, 1999. PMID: 10377174.
- Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, and Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 292: G1403-1410, 2007. PMID: 17234888.
- Chaudhuri A, Kolodecik TR, and Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 288: G235-243, 2005. PMID: 15458924.
- Chen H, Wild C, Zhou X, Ye N, Cheng X, and Zhou J. Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC). J Med Chem 57: 3651-3665, 2014. PMID: 24256330.
- Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, and Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics 5: 306-312, 2006. PMID: 16278343.
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, and Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625-628, 2000. PMID: 10915626.
- Chen Y, Harry A, Li J, Smit MJ, Bai X, Magnusson R, Pieroni JP, Weng G, and Iyengar R. Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation. Proc Natl Acad Sci U S A 94: 14100-14104, 1997. PMID: 9391159.
- Collen MJ, Sutliff VE, Pan GZ, and Gardner JD. Postreceptor modulation of action of VIP and secretin on pancreatic enzyme secretion by secretagogues that mobilize cellular calcium. AmJPhysiol 242: G423-G428, 1982. PMID: 6175231.
- Conti M, and Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481-511, 2007. PMID: 17376027.
- Cooper DM, Mons N, and Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374: 421-424, 1995. PMID: 7700350.
- Cumbay MG, and Watts VJ. Galphaq potentiation of adenylate cyclase type 9 activity through a Ca2+/calmodulin-dependent pathway. Biochem Pharmacol 69: 1247-1256, 2005. PMID: 15794946.
- Cumbay MG, and Watts VJ. Novel regulatory properties of human type 9 adenylate cyclase. J Pharmacol Exp Ther 310: 108-115, 2004. PMID: 14996950.
- De Lisle RC, and Howell GW. Evidence of heterotrimeric G-protein involvement in regulated exocytosis from permeabilized pancreatic acini. Pancreas 10: 374-381, 1995. PMID: 7792294.
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, and Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474-477, 1998. PMID: 9853756.
- Defer N, Best-Belpomme M, and Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400-416, 2000. PMID: 10966920.
- Dehaye JP, Gillard M, Poloczek P, Stievenart M, Winand J, and Christophe J. Effects of forskolin on adenylate cyclase activity and amylase secretion in the rat exocrine pancreas. J Cyclic Nucleotide Protein Phosphor Res 10: 269-280, 1985. PMID: 2410466.
- Derbyshire ER, and Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533-559, 2012. PMID: 22404633.
- DiFrancesco JC, and DiFrancesco D. Dysfunctional HCN ion channels in neurological diseases. Front Cell Neurosci 6: 174, 2015. PMID: 25805968.
- DiMagno MJ, Williams JA, Hao Y, Ernst SA, and Owyang C. Endothelial nitric oxide synthase is protective in the initiation of caerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 287: G80-87, 2004. PMID: 14962849.
- Drucker DJ. Glucagon-like peptides. Diabetes 47: 159-169, 1998. PMID: 9519708.
- Duan RD, Guo YJ, and Williams JA. Conversion to Ca2+-independent form of Ca2+/calmodulin protein kinase II in rat pancreatic acini. Biochem Biophys Res Commun 199: 368-373, 1994. PMID: 8123036.
- Feinstein PG, Schrader KA, Bakalyar HA, Tang WJ, Krupinski J, Gilman AG, and Reed RR. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proc Natl Acad Sci U S A 88: 10173-10177, 1991. PMID: 1719547.
- Ficker E, Jarolimek W, and Brown AM. Molecular determinants of inactivation and dofetilide block in ether a-go-go (EAG) channels and EAG-related K+ channels. Mol Pharmacol 60: 1343-1348, 2001. PMID: 11723241.
- Fields TA, and Casey PJ. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J 321 ( Pt 3): 561-571, 1997. PMID: 9032437.
- Forte LR, Jr. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104: 137-162, 2004. PMID: 15518884.
- Francis SH, Busch JL, Corbin JD, and Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525-563, 2010. PMID: 20716671.
- Gardner JD, Korman LY, Walker MD, and Sutliff VE. Effects of inhibitors of cyclic nucleotide phosphodiesterase on the actions of vasoactive intestinal peptide and secretin on pancreatic acini. Am J Physiol 242: G547-551, 1982. PMID: 6178297.
- Gardner JD, and Rottman AJ. Action of cholera toxin on dispersed acini from guinea pig pancreas. Biochim Biophys Acta 585: 250-265, 1979. PMID: 222350.
- Gardner JD, Sutliff VE, Walker MD, and Jensen RT. Effects of inhibitors of cyclic nucleotide phosphodiesterase on actions of cholecystokinin, bombesin, and carbachol on pancreatic acini. AmJPhysiol 245: G676-G680, 1983. PMID: 6195928.
- Giovannucci DR, Groblewski GE, Sneyd J, and Yule DI. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J Biol Chem 275: 33704-33711, 2000. PMID: 10887192.
- Gloerich M, and Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355-375, 2010. PMID: 20055708.
- Groblewski GE, Wagner AC, and Williams JA. Cyclosporin A inhibits Ca2+/calmodulin-dependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem 269: 15111-15117, 1994. PMID: 7515049.
- Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, and Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem 273: 22738-22744, 1998. PMID: 9712905.
- Gukovskaya A, and Pandol S. Nitric oxide production regulates cGMP formation and calcium influx in pancreatic acinar cells. Am J Physiol 266: G350-356, 1994. PMID: 8166275.
- Gurda GT, Guo L, Lee SH, Molkentin JD, and Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19: 198-206, 2008. PMID: 17978097.
- Harteneck C, Wedel B, Koesling D, Malkewitz J, Bohme E, and Schultz G. Molecular cloning and expression of a new alpha-subunit of soluble guanylyl cyclase. Interchangeability of the alpha-subunits of the enzyme. FEBS Lett 292: 217-222, 1991. PMID: 1683630.
- Haymovits A, and Scheele GA. Cellular cyclic nucleotides and enzyme secretion in the pancreatic acinar cell. Proc Natl Acad Sci U S A 73: 156-160, 1976. PMID: 174097.
- Heisler S. Forskolin potentiates calcium-dependent amylase secretion from rat pancreatic acinar cells. Can J Physiol Pharmacol 61: 1168-1176, 1983. PMID: 6196099.
- Heisler S, Kopelman H, Chabot JG, and Morel G. Atrial natriuretic factor and exocrine pancreas: effects on the secretory process. Pancreas 2: 243-251, 1987. PMID: 2442744.
- Herrmann S, Schnorr S, and Ludwig A. HCN channels--modulators of cardiac and neuronal excitability. Int J Mol Sci 16: 1429-1447, 2015. PMID: 25580535.
- Hetman JM, Soderling SH, Glavas NA, and Beavo JA. Cloning and characterization of PDE7B, a cAMP-specific phosphodiesterase. Proc Natl Acad Sci U S A 97: 472-476, 2000. PMID: 10618442.
- Ho JJ, Crawley S, Pan PL, Farrelly ER, and Kim YS. Secretion of MUC5AC mucin from pancreatic cancer cells in response to forskolin and VIP. Biochem Biophys Res Commun 294: 680-686, 2002. PMID: 12056823.
- Hofmann F, Bernhard D, Lukowski R, and Weinmeister P. cGMP regulated protein kinases (cGK). Handb Exp Pharmacol 137-162, 2009. PMID: 19089329.
- Holz GG, Kang G, Harbeck M, Roe MW, and Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol 577: 5-15, 2006. PMID: 16973695.
- Hou Y, Ernst SA, Heidenreich K, and Williams JA. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am J Physiol Gastrointest Liver Physiol 310: G26-33, 2016. PMID: 26542397.
- Husain SZ, Grant WM, Gorelick FS, Nathanson MH, and Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 292: G1594-1599, 2007. PMID: 17332472.
- Ideno N, Yamaguchi H, Ghosh B, Gupta S, Okumura T, Steffen DJ, Fisher CG, Wood LD, Singhi AD, Nakamura M, Gutkind JS, and Maitra A. GNAS(R201C) Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 155: 1593-1607 e1512, 2018. PMID: 30142336.
- Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, and Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 511 ( Pt 2): 407-422, 1998. PMID: 9706019.
- Ishiguro H, Steward MC, Lindsay AR, and Case RM. Accumulation of intracellular HCO3- by Na+-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495 ( Pt 1): 169-178, 1996. PMID: 8866360.
- Jaworek J, Jachimczak B, Bonior J, Kot M, Tomaszewska R, Karczewska E, Stachura J, Pawlik W, and Konturek SJ. Protective role of endogenous nitric oxide (NO) in lipopolysaccharide--induced pancreatic damage (a new experimental model of acute pancreatitis). J Physiol Pharmacol 51: 85-102, 2000. PMID: 10768853.
- Jaworek J, Jachimczak B, Tomaszewska R, Konturek PC, Pawlik WW, Sendur R, Hahn EG, Stachura J, and Konturek SJ. Protective action of lipopolysaccharidesin rat caerulein-induced pancreatitis: role of nitric oxide. Digestion 62: 1-13, 2000. PMID: 10899719.
- Jensen RT, and Gardner JD. Cyclic nucleotide-dependent protein kinase activity in acinar cells from guinea pig pancreas. Gastroenterology 75: 806-816, 1978. PMID: 212341.
- Jobling MG, Gotow LF, Yang Z, and Holmes RK. A mutational analysis of residues in cholera toxin A1 necessary for interaction with its substrate, the stimulatory G protein Gsalpha. Toxins (Basel) 7: 919-935, 2015. PMID: 25793724.
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, and Holz GG. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem 278: 8279-8285, 2003. PMID: 12496249.
- Karakhanova S, Golovastova M, Philippov PP, Werner J, and Bazhin AV. Interlude of cGMP and cGMP/protein kinase G type 1 in pancreatic adenocarcinoma cells. Pancreas 43: 784-794, 2014. PMID: 24826884.
- Kaupp UB, and Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769-824, 2002. PMID: 12087135.
- Kim SJ, and Greger R. Voltage-dependent, slowly activating K+ current (IKs) and its augmentation by carbachol in rat pancreatic acini. Pflugers Arch 438: 604-611, 1999. PMID: 10555556.
- Kim SJ, Kim JK, Pavenstadt H, Greger R, Hug MJ, and Bleich M. Regulation of slowly activating potassium current (IKs) by secretin in rat pancreatic acinar cells. J Physiol 535: 349-358, 2001. PMID: 11533128.
- Kimura T, Imamura K, Eckhardt L, and Schulz I. Ca2+-, phorbol ester-, and cAMP-stimulated enzyme secretion from permeabilized rat pancreatic acini. Am J Physiol 250: G698-708, 1986. PMID: 2422955.
- Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, and Steegborn C. Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proc Natl Acad Sci U S A 111: 3727-3732, 2014. PMID: 24567411.
- Kloeters O, Friess H, Giese N, Buechler MW, Cetin Y, and Kulaksiz H. Uroguanylin inhibits proliferation of pancreatic cancer cells. Scand J Gastroenterol 43: 447-455, 2008. PMID: 18365910.
- Koesling D, and Friebe A. Soluble guanylyl cyclase: structure and regulation. Rev Physiol Biochem Pharmacol 135: 41-65, 1999. PMID: 9932480.
- Kolodecik TR, Shugrue CA, Thrower EC, Levin LR, Buck J, and Gorelick FS. Activation of soluble adenylyl cyclase protects against secretagogue stimulated zymogen activation in rat pancreaic acinar cells. PLoS One 7: e41320, 2012. PMID: 22844459.
- Konturek SJ, Bilski J, Konturek PK, Cieszkowski M, and Pawlik W. Role of endogenous nitric oxide in the control of canine pancreatic secretion and blood flow. Gastroenterology 104: 896-902, 1993. PMID: 7680020.
- Kost CK, Jr., Herzer WA, Li PJ, and Jackson EK. Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 26: 449-455, 1999. PMID: 10386237.
- Kozasa T, Itoh H, Tsukamoto T, and Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A 85: 2081-2085, 1988. PMID: 3127824.
- Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, Slaughter C, Reed RR, and Gilman AG. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science 244: 1558-1564, 1989. PMID: 2472670.
- Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93: 700-709, 2003. PMID: 14563709.
- Kulaksiz H, and Cetin Y. Uroguanylin and guanylate cyclase C in the human pancreas: expression and mutuality of ligand/receptor localization as indicators of intercellular paracrine signaling pathways. J Endocrinol 170: 267-275, 2001. PMID: 11431160.
- Kulaksiz H, Schmid A, Honscheid M, Eissele R, Klempnauer J, and Cetin Y. Guanylin in the human pancreas: a novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system. Histochem Cell Biol 115: 131-145, 2001. PMID: 11444148.
- Kunapuli P, and Benovic JL. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A 90: 5588-5592, 1993. PMID: 7685906.
- Kurihara K, and Nakanishi N. Regulation of Na,K-ATPase by cAMP-dependent protein kinase anchored on membrane via A-kinase anchoring protein subtype, AKAP-150, in rat parotid gland. Ann N Y Acad Sci 986: 636-638, 2003. PMID: 12763907.
- LeBeau AP, Yule DI, Groblewski GE, and Sneyd J. Agonist-dependent phosphorylation of the inositol 1,4,5-trisphosphate receptor: A possible mechanism for agonist-specific calcium oscillations in pancreatic acinar cells. J Gen Physiol 113: 851-872, 1999. PMID: 10352035.
- Lee E, Gerlach U, Uhm DY, and Kim J. Inhibitory effect of somatostatin on secretin-induced augmentation of the slowly activating K+ current (IKs) in the rat pancreatic acinar cell. Pflugers Arch 443: 405-410, 2002. PMID: 11810210.
- Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, and Muallem S. Regulation of Cl-/ HCO3- exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J Biol Chem 274: 3414-3421, 1999. PMID: 9920885.
- Li H, Liu JP, and Robinson PJ. Multiple substrates for cGMP-dependent protein kinase from bovine aortic smooth muscle: purification of P132. J Vasc Res 33: 99-110, 1996. PMID: 8630352.
- Linder ME, and Gilman AG. G proteins. Sci Am 267: 56-61, 64-55, 1992. PMID: 1502510.
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, and Levin LR. Kinetic properties of "soluble" adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem 278: 15922-15926, 2003. PMID: 12609998.
- Love JA, and Szebeni K. Histochemistry and electrophysiology of cultured adult rabbit pancreatic neurons. Pancreas 18: 65-74, 1999. PMID: 9888662.
- Lu Z, Kolodecik TR, Karne S, Nyce M, and Gorelick F. Effect of ligands that increase cAMP on caerulein-induced zymogen activation in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 285: G822-828, 2003. PMID: 12881228.
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, and Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375-414, 2000. PMID: 10977868.
- Luo X, Ahn W, Muallem S, and Zeng W. Analyses of RGS protein control of agonist-evoked Ca2+ signaling. Methods Enzymol 389: 119-130, 2004. PMID: 15313563.
- Ventimiglia MS, Najenson AC, Rodríguez MR, Davio CA, Vatta MS, and Bianciotti LG. Natriuretic Peptides and Their Receptors. Pancreapedia: Exocrine Pancreas Knowledge Base. 2011. DOI: 10.3998/panc.2011.36.
- Ma H, Butler MR, Thapa A, Belcher J, Yang F, Baehr W, Biel M, Michalakis S, and Ding XQ. cGMP/Protein Kinase G Signaling Suppresses Inositol 1,4,5-Trisphosphate Receptor Phosphorylation and Promotes Endoplasmic Reticulum Stress in Photoreceptors of Cyclic Nucleotide-gated Channel-deficient Mice. J Biol Chem 290: 20880-20892, 2015. PMID: 26124274.
- Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, and Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science 238: 675-678, 1987. PMID: 2823385.
- Matozaki T, Sakamoto C, Nagao M, Nishizaki H, and Baba S. G protein in stimulation of PI hydrolysis by CCK in isolated rat pancreatic acinar cells. Am J Physiol 255: E652-659, 1988. PMID: 2461094.
- Matrougui K, Maclouf J, Levy BI, and Henrion D. Impaired nitric oxide- and prostaglandin-mediated responses to flow in resistance arteries of hypertensive rats. Hypertension 30: 942-947, 1997. PMID: 9336397.
- Matsushita K, Okabayashi Y, Hasegawa H, Koide M, Kido Y, Okutani T, Sugimoto Y, and Kasuga M. In vitro inhibitory effect of somatostatin on secretin action in exocrine pancreas of rats. Gastroenterology 104: 1146-1152, 1993. PMID: 7681794.
- Mersin H, Irkin F, Berberoglu U, Gulben K, Ozdemir H, and Onguru O. The selective inhibition of type IV phosphodiesterase attenuates the severity of the acute pancreatitis in rats. Dig Dis Sci 54: 2577-2582, 2009. PMID: 19117125.
- Moglich A, Ayers RA, and Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17: 1282-1294, 2009. PMID: 19836329.
- Morley DJ, Hawley DM, Ulbright TM, Butler LG, Culp JS, and Hodes ME. Distribution of phosphodiesterase I in normal human tissues. J Histochem Cytochem 35: 75-82, 1987. PMID: 3025290.
- Moustafa A, Sakamoto KQ, and Habara Y. Nitric oxide stimulates IP3 production via a cGMP/ PKG-dependent pathway in rat pancreatic acinar cells. Jpn J Vet Res 59: 5-14, 2011. PMID: 21476485.
- Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 355: 2003-2011, 2006. PMID: 17093251.
- Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR, and Makhlouf GM. Gi-1/Gi-2-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am J Physiol Gastrointest Liver Physiol 278: G974-980, 2000. PMID: 10859228.
- Nelson EJ, Hellevuo K, Yoshimura M, and Tabakoff B. Ethanol-induced phosphorylation and potentiation of the activity of type 7 adenylyl cyclase. Involvement of protein kinase C delta. J Biol Chem 278: 4552-4560, 2003. PMID: 12454008.
- Nomura M, Beltran C, Darszon A, and Vacquier VD. A soluble adenylyl cyclase from sea urchin spermatozoa. Gene 353: 231-238, 2005. PMID: 15978750.
- O'Sullivan AJ, and Jamieson JD. Protein kinase A modulates Ca2+- and protein kinase C-dependent amylase release in permeabilized rat pancreatic acini. Biochem J 287 ( Pt 2): 403-406, 1992. PMID: 1280101.
- Ohnishi H, Mine T, and Kojima I. Inhibition by somatostatin of amylase secretion induced by calcium and cyclic AMP in rat pancreatic acini. Biochem J 304 ( Pt 2): 531-536, 1994. PMID: 7528010.
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, and Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2: 805-811, 2000. PMID: 11056535.
- Pagano M, and Anand-Srivastava MB. Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem 276: 22064-22070, 2001. PMID: 11303026.
- Palaparti A, Li Y, and Anand-Srivastava MB. Inhibition of atrial natriuretic peptide (ANP) C receptor expression by antisense oligodeoxynucleotides in A10 vascular smooth-muscle cells is associated with attenuation of ANP-C-receptor-mediated inhibition of adenylyl cyclase. Biochem J 346 Pt 2: 313-320, 2000. PMID: 10677348.
- Pandey KN. Natriuretic peptides and their receptors. Peptides 26: 899-900, 2005. PMID: 15885851.
- Pandol SJ, Gukovskaya A, Bahnson TD, and Dionne VE. Cellular mechanisms mediating agonist-stimulated calcium influx in the pancreatic acinar cell. Ann N Y Acad Sci 713: 41-48, 1994. PMID: 8185204.
- Pandol SJ, and Schoeffield-Payne MS. Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem 265: 12846-12853, 1990. PMID: 2165487.
- Pascua P, Garcia M, Fernandez-Salazar MP, Hernandez-Lorenzo MP, Calvo JJ, Colledge WH, Case RM, Steward MC, and San Roman JI. Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflugers Arch 459: 203-214, 2009. PMID: 19655163.
- Pierce KL, Premont RT, and Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639-650, 2002. PMID: 12209124.
- Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, and Hanoune J. Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem 271: 13900-13907, 1996. PMID: 8662814.
- Quinn SN, Graves SH, Dains-McGahee C, Friedman EM, Hassan H, Witkowski P, and Sabbatini ME. Adenylyl cyclase 3/adenylyl cyclase-associated protein 1 (CAP1) complex mediates the anti-migratory effect of forskolin in pancreatic cancer cells. Mol Carcinog 56: 1344-1360, 2017. PMID: 27891679.
- Ramos-Alvarez I, Lee L, and Jensen RT. Cyclic AMP-dependent protein kinase A and EPAC mediate VIP and secretin stimulation of PAK4 and activation of Na+, K+-AT Pase in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 316: G263-G277, 2019. PMID: 30520694.
- Sabbatini ME. Natriuretic peptides as regulatory mediators of secretory activity in the digestive system. Regul Pept 154: 5-15, 2009. PMID: 19233231.
- Sabbatini ME, Chen X, Ernst SA, and Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem 283: 23884-23894, 2008. PMID: 18577515.
- Sabbatini ME, D'Alecy L, Lentz SI, Tang T, and Williams JA. Adenylyl cyclase 6 mediates the action of cyclic AMP-dependent secretagogues in mouse pancreatic exocrine cells via protein kinase A pathway activation. J Physiol 591: 3693-3707, 2013. PMID: 23753526.
- Sabbatini ME, Gorelick F, and Glaser S. Adenylyl cyclases in the digestive system. Cell Signal 26: 1173-1181, 2014. PMID: 24521753.
- Sabbatini ME, Rodriguez M, di Carlo MB, Davio CA, Vatta MS, and Bianciotti LG. C-type natriuretic peptide enhances amylase release through NPR-C receptors in the exocrine pancreas. Am J Physiol Gastrointest Liver Physiol 293: G987-994, 2007. PMID: 17702953.
- Sabbatini ME, Rodriguez MR, Dabas P, Vatta MS, and Bianciotti LG. C-type natriuretic peptide stimulates pancreatic exocrine secretion in the rat: role of vagal afferent and efferent pathways. Eur J Pharmacol 577: 192-202, 2007. PMID: 17900562.
- Sabbatini ME, Vatta MS, Vescina C, Gonzales S, Fernandez B, and Bianciotti LG. NPR-C receptors are involved in C-type natriuretic peptide response on bile secretion. Regul Pept 116: 13-20, 2003. PMID: 14599710.
- Sadana R, and Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17: 5-22, 2009. PMID: 18948702.
- Sadana R, and Dessauer CWT. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17: 5-22, 2009. PMID: 18948702.
- Saino T, and Watson EL. Inhibition of serine/threonine phosphatase enhances arachidonic acid-induced [Ca2+]i via protein kinase A. Am J Physiol Cell Physiol 296: C88-96, 2009. PMID: 18987253.
- Schafer C, Steffen H, Krzykowski KJ, Goke B, and Groblewski GE. CRHSP-24 phosphorylation is regulated by multiple signaling pathways in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 285: G726-734, 2003. PMID: 12801884.
- Schnefel S, Profrock A, Hinsch KD, and Schulz I. Cholecystokinin activates Gi1-, Gi2-, Gi3- and several Gs-proteins in rat pancreatic acinar cells. Biochem J 269: 483-488, 1990. PMID: 2117441.
- Schneider EH, and Seifert R. Report on the Third Symposium "cCMP and cUMP as New Second Messengers". Naunyn Schmiedebergs Arch Pharmacol 388: 1-3, 2015. PMID: 25471064.
- Seamon KB, and Daly JW. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res 20: 1-150, 1986. PMID: 3028083.
- Seifert R, Schneider EH, and Bahre H. From canonical to non-canonical cyclic nucleotides as second messengers: pharmacological implications. Pharmacol Ther 148: 154-184, 2015. PMID: 25527911.
- Seino S, and Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303-1342, 2005. PMID: 16183914.
- Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2019. CA Cancer J Clin 69: 7-34, 2019. PMID: 30620402.
- Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA Cancer J Clin 70: 7-30, 2020. PMID: 31912902.
- Sindic A, and Schlatter E. Renal electrolyte effects of guanylin and uroguanylin. Curr Opin Nephrol Hypertens 16: 10-15, 2007. PMID: 17143065.
- Singh M. Role of cyclic adenosine monophosphate in amylase release from dissociated rat pancreatic acini. J Physiol 331: 547-555, 1982. PMID: 6185668.
- Singh P, Asada I, Owlia A, Collins TJ, and Thompson JC. Somatostatin inhibits VIP-stimulated amylase release from perifused guinea pig pancreatic acini. Am J Physiol 254: G217-223, 1988. PMID: 2450469.
- Sinnarajah S, Dessauer CW, Srikumar D, Chen J, Yuen J, Yilma S, Dennis JC, Morrison EE, Vodyanoy V, and Kehrl JH. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature 409: 1051-1055, 2001. PMID: 11234015.
- Steegborn C. Structure, mechanism, and regulation of soluble adenylyl cyclases - similarities and differences to transmembrane adenylyl cyclases. Biochim Biophys Acta 1842: 2535-2547, 2014. PMID: 25193033.
- Steegborn C, Litvin TN, Levin LR, Buck J, and Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol 12: 32-37, 2005. PMID: 15619637.
- Straub SV, Giovannucci DR, Bruce JI, and Yule DI. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by Peptide agonists in pancreatic acinar cells. J Biol Chem 277: 31949-31956, 2002. PMID: 12065595.
- Stryjek-Kaminska D, Piiper A, and Zeuzem S. EGF inhibits secretagogue-induced cAMP production and amylase secretion by Gi proteins in pancreatic acini. Am J Physiol 269: G676-682, 1995. PMID: 7491958.
- Sunahara RK, and Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2: 168-184, 2002. PMID: 14993377.
- Taki K, Ohmuraya M, Tanji E, Komatsu H, Hashimoto D, Semba K, Araki K, Kawaguchi Y, Baba H, and Furukawa T. GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 35: 2407-2412, 2016. PMID: 26257060.
- Takumi T, Ohkubo H, and Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science 242: 1042-1045, 1988. PMID: 3194754.
- Tang WJ, and Gilman AG. Adenylyl cyclases. Cell 70: 869-872, 1992. PMID: 1525824.
- Taylor SS, Zhang P, Steichen JM, Keshwani MM, and Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 1834: 1271-1278, 2013. PMID: 23535202.
- Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H, and Seino S. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics 78: 91-98, 2001. PMID: 11707077.
- Ulrich CD, 2nd, Holtmann M, and Miller LJ. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology 114: 382-397, 1998. PMID: 9453500.
- Wang R, Li J, and Rosenberg L. Factors mediating the transdifferentiation of islets of Langerhans to duct epithelial-like structures. J Endocrinol 171: 309-318, 2001. PMID: 11691651.
- Warth R, Garcia Alzamora M, Kim JK, Zdebik A, Nitschke R, Bleich M, Gerlach U, Barhanin J, and Kim SJ. The role of KCNQ1/KCNE1 K+ channels in intestine and pancreas: lessons from the KCNE1 knockout mouse. Pflugers Arch 443: 822-828, 2002. PMID: 11889581.
- Weber IT, Gilliland GL, Harman JG, and Peterkofsky A. Crystal structure of a cyclic AMP-independent mutant of catabolite gene activator protein. J Biol Chem 262: 5630-5636, 1987. PMID: 3032940.
- Weyand I, Godde M, Frings S, Weiner J, Muller F, Altenhofen W, Hatt H, and Kaupp UB. Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature 368: 859-863, 1994. PMID: 7512693.
- Willems PH, Tilly RH, and de Pont JJ. Pertussis toxin stimulates cholecystokinin-induced cyclic AMP formation but is without effect on secretagogue-induced calcium mobilization in exocrine pancreas. Biochim Biophys Acta 928: 179-185, 1987. PMID: 2436669.
- Williams JA, Yule, D.I. Stimulus-secretion coupling in pancreatic acinar cells. In: Physiology of the Gastrointestinal Tract. 5TH Ed. Vol 2. edited by Johnson LR. London. Elsevier BV, 2012, p. 1361-1398. DOI: 10.1016/B978-0-12-382026-6.00050.6.
- Willoughby D, and Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965-1010, 2007. PMID: 17615394.
- Winger JA, and Marletta MA. Expression and characterization of the catalytic domains of soluble guanylate cyclase: interaction with the heme domain. Biochemistry 44: 4083-4090, 2005. PMID: 15751985.
- Wrenn RW, Currie MG, and Herman LE. Nitric oxide participates in the regulation of pancreatic acinar cell secretion. Life Sci 55: 511-518, 1994. PMID: 7518887.
- Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Jr., Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, and Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 3: 92ra66, 2011. PMID: 21775669.
- Xie T, Chen M, and Weinstein LS. Pancreas-specific Gsalpha deficiency has divergent effects on pancreatic alpha- and beta-cell proliferation. J Endocrinol 206: 261-269, 2010. PMID: 20543009.
- Yadav D, and Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 7: 131-145, 2010. PMID: 20125091.
- Yamamoto T, Yamato E, Taniguchi H, Shimoda M, Tashiro F, Hosoi M, Sato T, Fujii S, and Miyazaki JI. Stimulation of cAMP signalling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia 49: 2359-2367, 2006. PMID: 16896938.
- Yan SZ, Huang ZH, Andrews RK, and Tang WJ. Conversion of forskolin-insensitive to forskolin-sensitive (mouse-type IX) adenylyl cyclase. Mol Pharmacol 53: 182-187, 1998. PMID: 9463474.
- Yan SZ, Huang ZH, Shaw RS, and Tang WJ. The conserved asparagine and arginine are essential for catalysis of mammalian adenylyl cyclase. J Biol Chem 272: 12342-12349, 1997. PMID: 9139678.
- Yang H, Lee CJ, Zhang L, Sans MD, and Simeone DM. Regulation of transforming growth factor beta-induced responses by protein kinase A in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 295: G170-G178, 2008. PMID: 18467503.
- Yang WP, Levesque PC, Little WA, Conder ML, Shalaby FY, and Blanar MA. KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias. Proc Natl Acad Sci U S A 94: 4017-4021, 1997. PMID: 9108097.
- Yoshida H, Tsunoda Y, and Owyang C. Effect of uncoupling NO/cGMP pathways on carbachol- and CCK-stimulated Ca2+ entry and amylase secretion from the rat pancreas. Pflugers Arch 434: 25-37, 1997. PMID: 9094253.
- Yuen PS, Potter LR, and Garbers DL. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry 29: 10872-10878, 1990. PMID: 1980215.
- Yurkanis Bruice P. The Chemistry of Nucleic Acids. In: Organic Chemistry, by: Bruice PY. Pearson Education Inc.p: 1155-1181. ISBN-13: 978-0134074580.
- Zaccolo M, and Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res 100: 1569-1578, 2007. PMID: 17556670.
- Zhang D, Ma QY, Hu HT, and Zhang M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther 10: 19-29, 2010. PMID: 20424515.
- Zhang G, Liu Y, Ruoho AE, and Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature 386: 247-253, 1997. PMID: 9069282.
- Zimmerman NP, Roy I, Hauser AD, Wilson JM, Williams CL, and Dwinell MB. Cyclic AMP regulates the migration and invasion potential of human pancreatic cancer cells. Mol Carcinog 54: 203-215, 2015. PMID: 24115212.
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, and Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82-84, 2003. PMID: 12475901.
- Zippin JH, Levin LR, and Buck J. CO2/HCO3--responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab 12: 366-370, 2001. PMID: 11551811.