Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2020.08
| Attachment | Size |
|---|---|
| 1.92 MB |
Abstract:
Pancreatic stellate cells (PSCs) are resident cells of the pancreas, found in both the exocrine and endocrine parts of the gland. Over the two decades since these cells were first isolated and cultured from rodent and human pancreas, research in this area has progressed at a rapid rate. Our knowledge of PSC biology in both health and disease has increased significantly. In health, PSCs are known to not only play a role in regulating normal extracellular matrix turnover but are also thought to have progenitor cell functions as well as a role in innate immunity. The critical roles of PSCs in inflammatory as well as malignant disease of the pancreas are now being increasingly elucidated. An improved understanding of PSC biology and of the interactions of PSCs with other pancreatic cells provides a strong platform for the development of novel therapeutic approaches for notoriously hard to treat diseases of the pancreas such as chronic pancreatitis and pancreatic cancer.
I. INTRODUCTION
Pancreatic fibrosis is a well-recognized histopathological feature of two major diseases of the pancreas – chronic pancreatitis and pancreatic cancer. In health, the process of fibrogenesis is a well-regulated dynamic process which is necessary for regular turnover of extracellular matrix (ECM) that allows remodeling and maintenance of normal pancreatic architecture. However, during injury, the equilibrium between production and degradation of fibrous tissue is disrupted leading to excessive deposition of extracellular matrix proteins resulting in fibrosis.
Pancreatic stellate cells (PSCs) are now considered to be the key contributors of pancreatic fibrosis (5, 11, 135). These cells were first observed by Watari et al. (144) in 1982, and later confirmed by Ikejiri (54) in 1990. The development of isolation and culture methods for PSCs in 1998 helped to unravel the mechanisms involved in the process of pancreatic fibrogenesis (5, 11) and also helped researchers to investigate the functions of these cells both in health and disease.
II. PANCREATIC STELLATE CELLS (PSCs)
A. PSCs in health
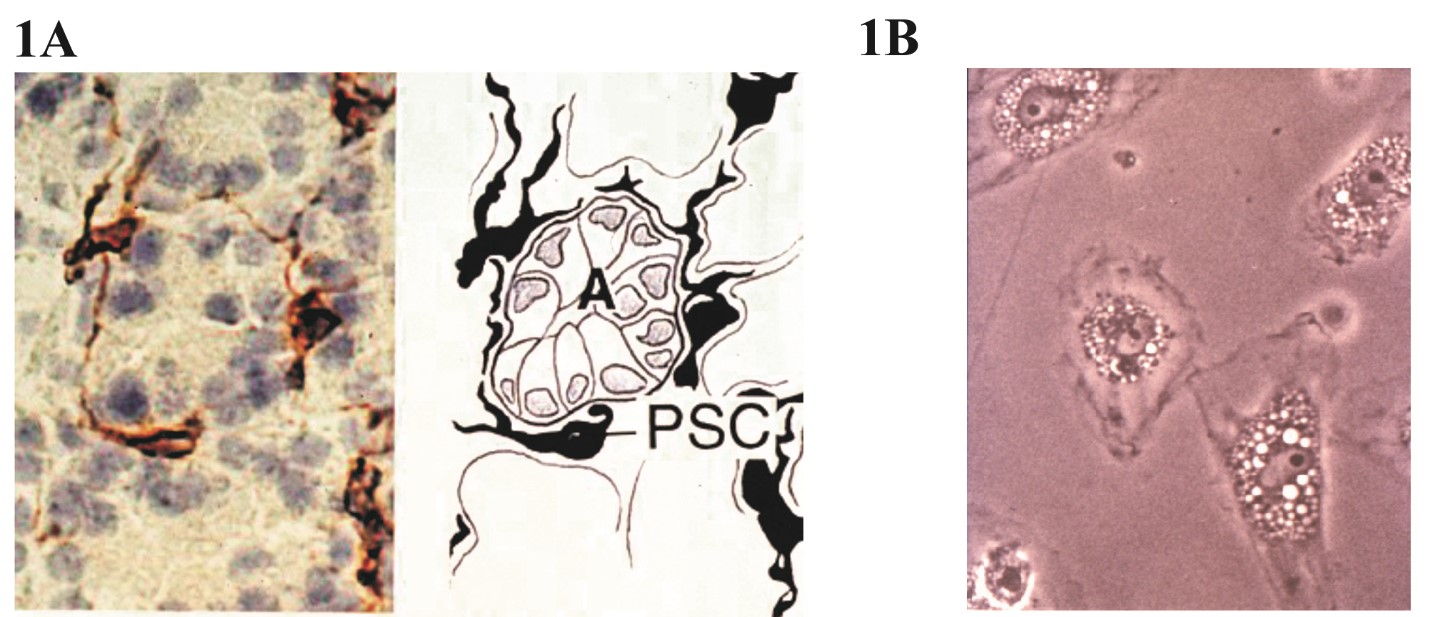
In a healthy pancreas, PSCs make up 4-7% (5) of the total parenchyma and are located around the basolateral aspects of the acinar cells (Figure 1A). PSCs are also found around small pancreatic ducts (5, 11), pancreatic islets (163) and blood vessels. PSCs in their native state express abundant vitamin A (retinoids) containing droplets in their cytoplasm which is a characteristic feature of the “stellate cell system” in the body (138). The buoyancy imparted by the vitamin A containing vesicles was utilized by Apte et al. (5) to develop a density gradient centrifugation method for the isolation of quiescent PSCs from the pancreas. In early culture, PSCs are polygonal in shape with abundant lipid droplets in the cytoplasm (Figure 1B) and express stellate cell selective markers such as desmin, glial fibrillary acidic protein (GFAP), nestin, neural cell adhesion molecule, nerve growth factor, and synemin (34, 167). Due to the expression of neural markers, PSCs were initially thought to be of neuroectodermal origin. However, lineage tracing studies with hepatic stellate cells (counterparts of PSCs in the liver) have confirmed a mesenchymal origin for these cells (9). The fact that a proportion of PSCs are replenished from bone marrow further supports a mesenchymal origin for PSCs (120).
Figure 1. (A) Normal rat pancreatic stellate cells stained for cytoskeletal protein desmin. Photomicrograph showing a normal rat pancreatic section immunostained for the stellate cell selective marker desmin (left) and a corresponding line diagram (right). Desmin-positive (brown) PSCs can be seen surrounding the basolateral aspects of pancreatic acini. (B) PSCs in an early culture showing a typical flattened polygonal shape with abundant vitamin A-containing lipid droplets in the cytoplasm. Source: Apte et al. 1998 (5). (Reproduced with permission from BMJ Publishing Group Ltd.)
Islet stellate cells (ISCs) are similar to but differ in certain aspects with PSCs. ISCs have fewer vitamin-A containing droplets and undergo rapid activation compared to PSCs. Upon activation, they express more α-SMA but have reduced rates of proliferation and migration compared to exocrine PSCs (163).
PSCs are the primary producers of ECM proteins such as collagen I–IV, fibronectin, and laminin. At the same time, they also produce ECM degrading enzymes like matrix metalloproteinases (MMP) and their inhibitors - tissue inhibitors of matrix metalloproteinases (TIMPs) (101). In the normal pancreas, these enzymes maintain an equilibrium between the deposition and degradation of ECM proteins. However, it is now recognised that PSCs have functions beyond ECM remodeling in the normal pancreas. PSCs express receptors for the secretagogue cholecystokinin (CCK1 and CCK2) (14) and have the capacity to synthesize the neurotransmitter acetylcholine (ACh). Upon exposure to physiological levels of CCK, PSCs have been shown to secrete ACh which, in turn, acts on the muscarinic receptors on acinar cells and induces amylase secretion. (102). PSCs also play an important role in innate immunity through the expression of Toll-like receptors (TLRs) 2, 3, 4 and 5 (78) which can recognise pathogen-associated molecular patterns (PAMPs), alarmins and endogenous molecules released by tissue damage (DAMPs). These cells also possess the ability to phagocytose polymorphonuclear neutrophils and cell debris, which is mediated by CD36 via peroxisome proliferator-activated receptor γ (116). PSCs may also possess progenitor-like capabilities since they express several stem cell markers, including CD133, SOX9, nestin, and GDF3 (64, 83). They can differentiate into other cell types including insulin-secreting cells under the influence of relevant growth factors (14, 41, 83, 88, 118).
B. PSCs in disease
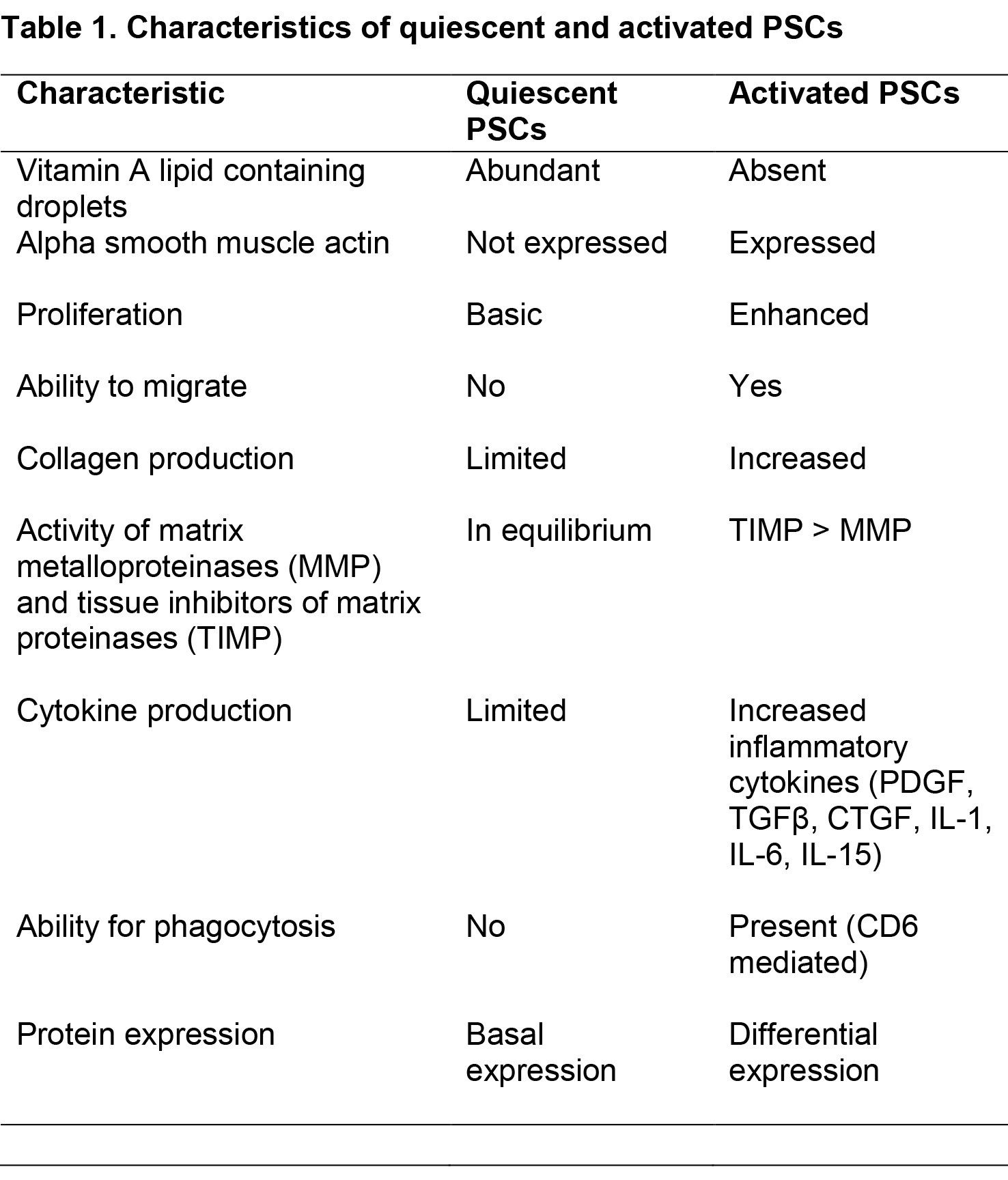
In the event of pancreatic injury, PSCs are activated, leading to loss of the vitamin A-containing lipid droplets and transform to a myofibroblast-like phenotype that expresses alpha smooth muscle actin (αSMA), fibroblast activation protein-α (FAP-α), fibroblast specific protein-1 (FSP-1), and fibrinogen (34). These cells also exhibit increased proliferation, migration, and ECM synthesis (4, 44). Activated PSCs produce collagenous stroma during pancreatic injury (8, 145, 152) which overwhelms the ECM degrading capacity of MMPs, leading to fibrosis. A comparison of resting and activated PSCs is given in Table 1.
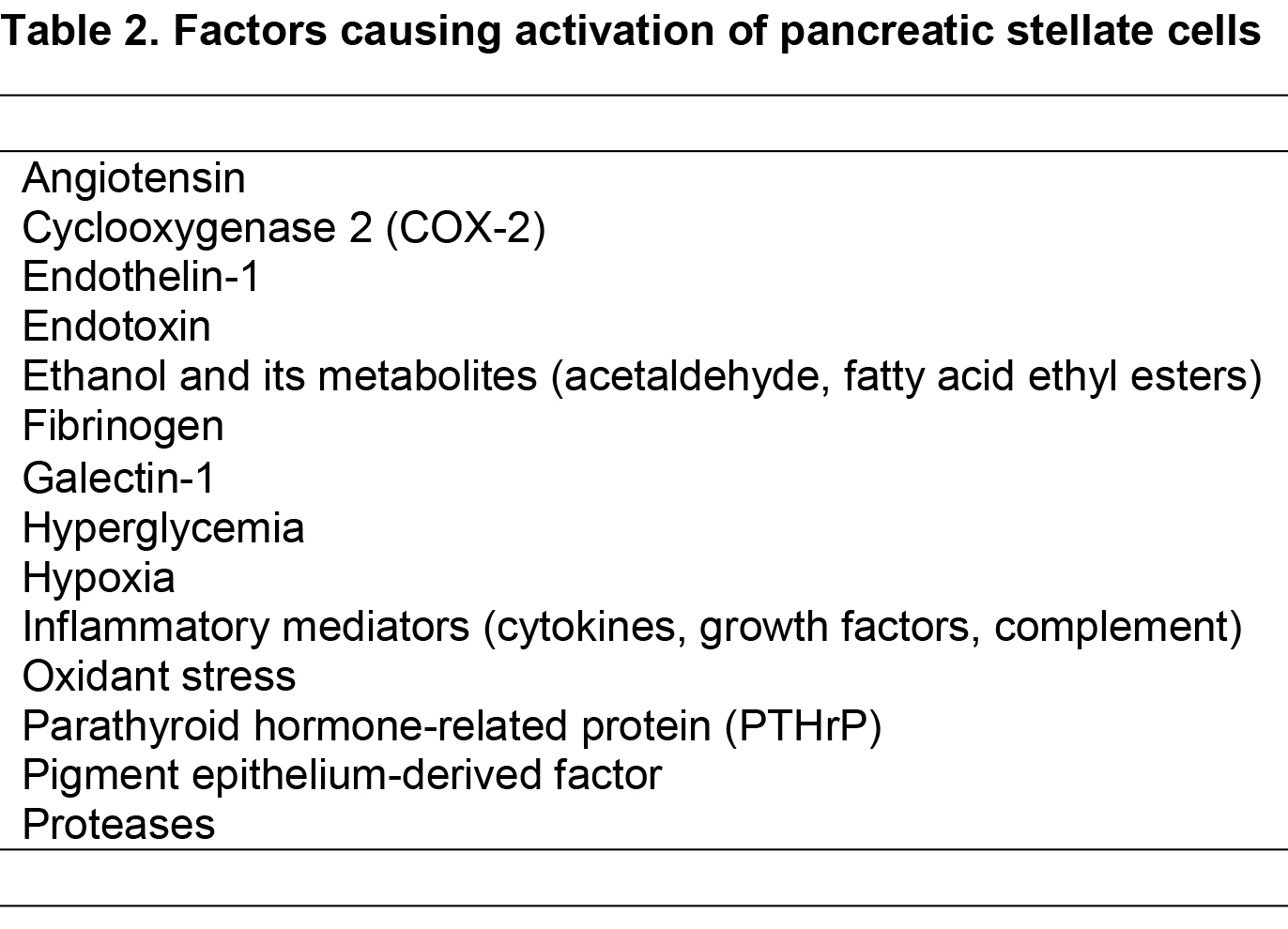
PSCs can be activated by several factors such as proinflammatory cytokines (87), oxidant stress (20, 130), ethanol and its metabolites, acetaldehyde and fatty acid ethyl esters (3, 80), fatty acids (oleate) (13) and endotoxins (137) (Table 2). In addition, in the setting of pancreatic cancer, factors such as acidosis (142), hypoxia (77), increased interstitial pressure (105) and hyperglycemia (92) are also involved in the activation of PSCs. Recently, epigenetic modifications (increased acetylation of histones) have also been shown to play an important role in the activation of PSCs and collagen synthesis (65). In addition to being activated by exogenous factors, PSCs are capable of sustaining their own activation (even in the absence of the initial triggers) through the production of cytokines such as (transforming growth factor beta [TGFβ], connective tissue growth factor [CTGF], interleukins 6, 7, 8 and 15, CXCR1, monocyte chemotactic protein 1 [MCP1], and regulated-on- activation- normal T-cell expressed-and-secreted [RANTES]) (2, 57, 112). These cytokines act via corresponding receptors on the PSCs themselves (autocrine effects) leading to a state of perpetual activation, which further facilitates pathological fibrosis.
Several pathways that mediate PSC activation have now been identified (summarized in Table 3). A number of these pathways interact with each other leading to significant redundancy in terms of the modulation of PSC activation (81, 85). Furthermore, studies have also shown that numerous signaling pathways can converge causing aberration of a common secondary messenger, namely, sustained increase in intracellular calcium within the PSCs (42, 48, 147). Recently microRNAs, (small noncoding RNAs), which are implicated in cell functions such as proliferation, differentiation, apoptosis, and protein synthesis, have been shown to differentially express between quiescent and activated PSCs (79). Other studies have implicated miR15b and 16 (known to regulate PSC apoptosis (113) and miR21 as possible cofactors in connective tissue growth factor (CCN2)-mediated PSC activation (22).
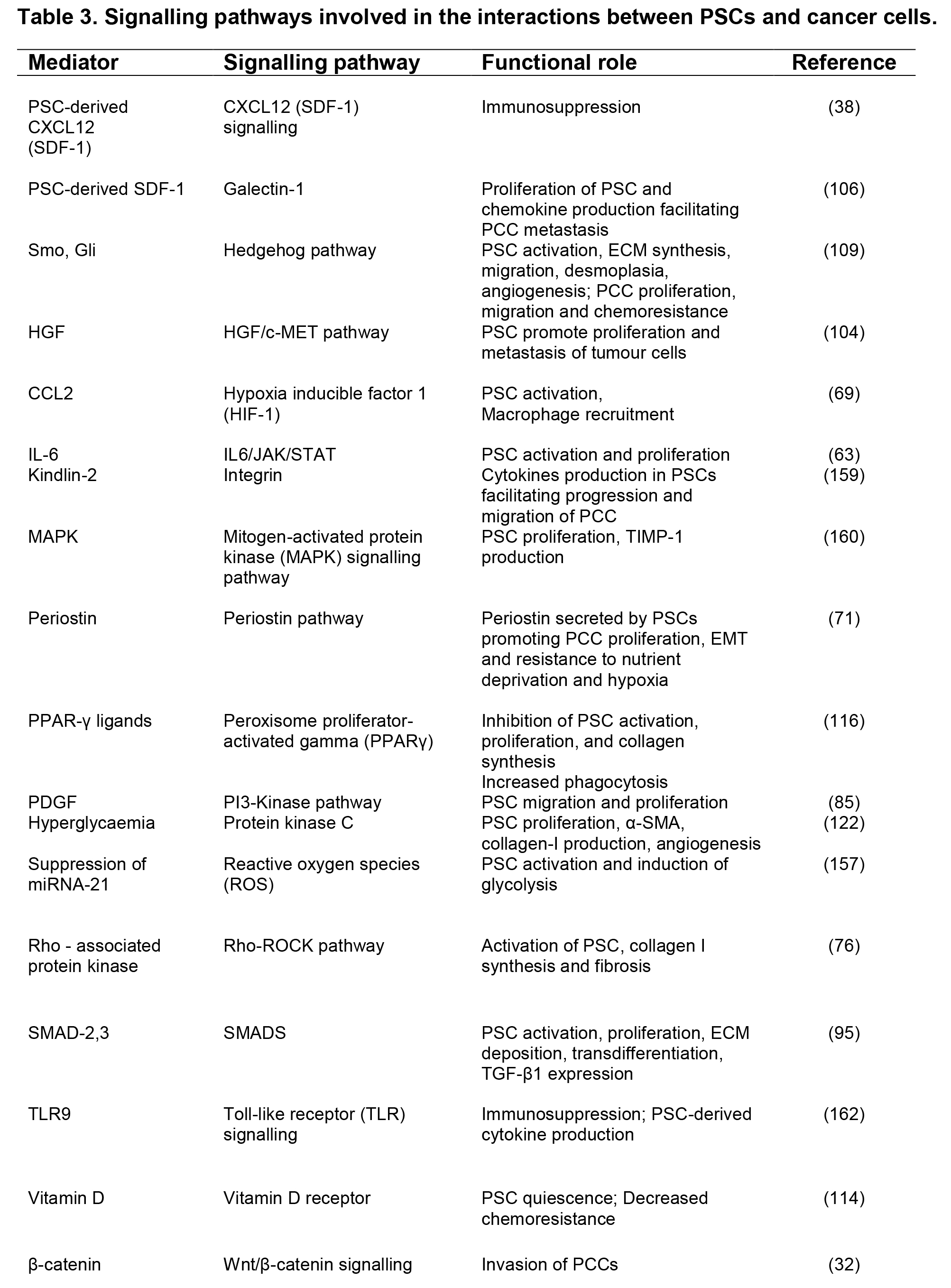
Table 3. Signalling pathways involved in the interactions between PSCs and cancer cells. α-SMA, alpha smooth muscle actin; c-MET, tyrosine-protein kinase of Met; CCL, chemokine ligand; CXCL, chemotactic cytokine ligand; ECM, extracellular matrix; JAK/STAT, Janus kinase/signal transducers and activators of transcription; HGF, hepatocyte growth factor; PSC, pancreatic stellate cells; PCC, pancreatic cancer cells; SDF, stromal derived factor; PDGF, platelet-derived growth factor; SMAD, small worm mothers against decapentaplegic.
The past two decades have seen a considerable improvement in our understanding of the role of PSCs in pancreatic inflammation (both acute and chronic pancreatitis) as well as pancreatic cancer. Acute pancreatitis is usually a self-limiting disease where PSCs aid remodeling and repair of tissue architecture, while both chronic pancreatitis and pancreatic cancer are characterized by pathological accumulation of fibrous tissue.
III. ACUTE PANCREATITIS
Acute pancreatitis is a condition which results from acute inflammation of the pancreatic parenchyma, ranging from mild inflammation to extensive pancreatic necrosis. Most of the cases are mild and self- limiting with a mortality rate <1%. The most common causes of acute pancreatitis are gallstones and alcohol excess (33).
In response to inflammatory cytokines and chemokines secreted by damaged acinar cells and inflammatory cells, PSCs are activated and proliferate early in acute pancreatitis (26). The majority of the increased numbers of activated PSCs, in the inflamed areas, are a result of local proliferation of resident PSCs. However, a small proportion (7–18%) are derived from circulating bone marrow cells (120). Activated PSCs secrete increased ECM proteins that act as a scaffold for regenerating ductal and acinar cells during the repair process. These ECM proteins are also important for differentiation and cell growth via integrin mediated interactions between cell membranes and the surrounding matrix (16). Indeed, using β1-integrin knockout animals, it has been shown that the lack of integrin-mediated interactions results in decreased ECM production by PSCs and increased apoptosis and decreased proliferation of acinar cells, thereby impeding pancreatic repair (108).
PSCs may also play an important role in remodeling and regeneration after acute necrotizing pancreatitis. Zimmerman et al. (168) observed that pilot ductules that originate from hypercellular regenerative spheres (islands of vascular granulation tissue, ductular cells, stellate cells, and residual lobular elements) grow outwards in close association with activated PSCs. The authors have suggested that these PSCs may be able to support differentiation of cells to mature duct and acinar cells, thereby playing a role in the reconstitution of the cell population after acute injury. The process of regeneration also requires removal of excess ECM, a task that is aided by the fibrinolytic activity of PSCs via the production of matrix degrading enzymes (MMPs).
IV. CHRONIC PANCREATITIS
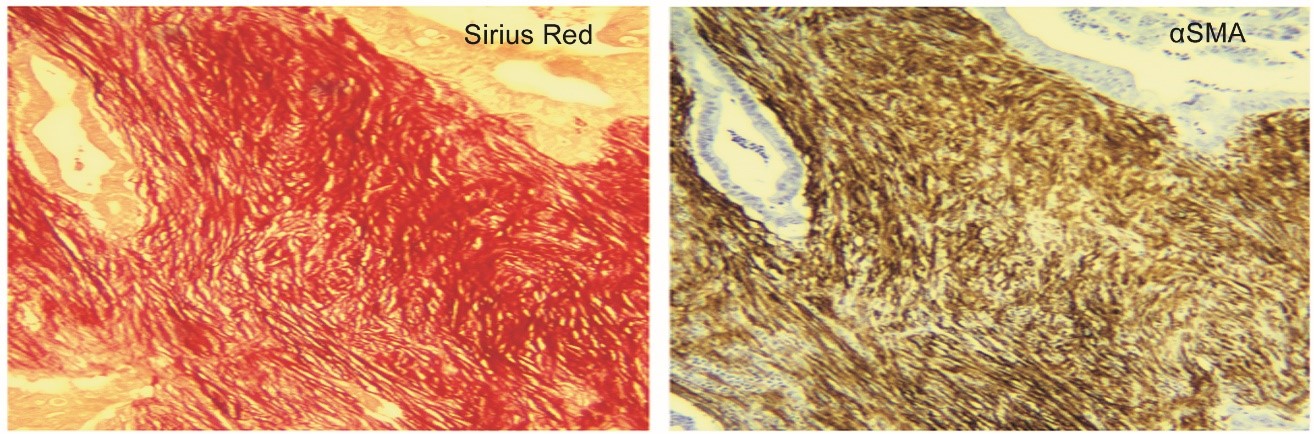
Chronic pancreatitis is a progressive disease characterized by fibrosis, acinar cell loss, distorted ducts, and inflammatory cell infiltration, eventually leading to significant exocrine and endocrine dysfunction (62). (Figure 2) The presence of activated PSCs was confirmed in the areas of fibrosis using dual staining techniques (Sirius Red for collagen and immunostaining for αSMA) (44) (Figure 3), while dual staining for αSMA and procollagen mRNA has shown that activated PSCs are the predominant source of collagen I in the fibrotic areas (44) (Figure 4). Interestingly, the fibrosis of chronic pancreatitis was found not to be limited to the exocrine parenchyma. Activated PSCs have been demonstrated within and surrounding pancreatic islets and are co-localized in fibrotic areas around the islets in diabetic Goto–Kakizaki rats (163) and db/db mice (obesity, diabetes, and dyslipidemia model due to leptin receptor deficiency) (150). Additionally, activated PSCs can inhibit insulin secretion and cause beta cell apoptosis, effects which are further aggravated by hyperglycemia (60). These findings suggest that PSCs can modulate islet cell function, suggesting a direct role for these cells in the diabetes of chronic pancreatitis.
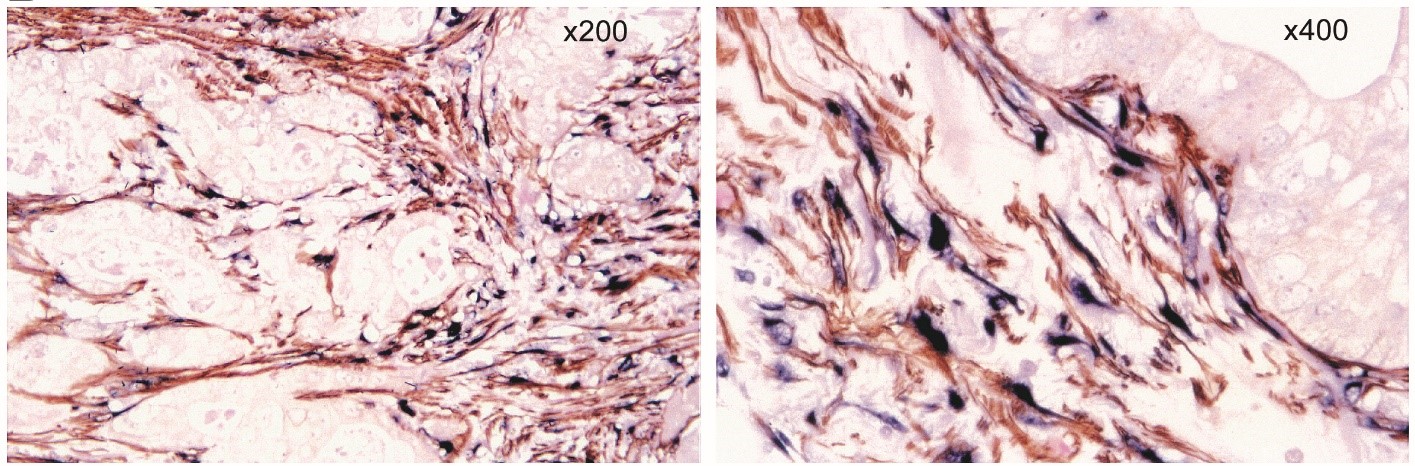
Figure 2. Activated PSCs in chronic pancreatitis. A section from a patient with chronic pancreatitis showing colocalization of staining for the PSC activation marker alpha smooth muscle actin (αSMA, brown) and collagen (using Sirius Red) (red) in fibrotic areas of the pancreas. Source: Haber et al. 1999 (44). (Reproduced with permission of Elsevier).
Figure 3. Colocalization of collagen and alpha SMA staining in pancreatic cancer. A representative pair of serial paraffin sections of the pancreas from a patient with pancreatic cancer demonstrating that stromal areas exhibit strong positive staining for collagen as well as for alpha SMA, indicative of the presence of activated PSCs in the desmoplastic reaction in pancreatic cancer. Original magnification, × 100. Source: Apte et al. 2004 (6). (Reproduced with permission of Wolter Kluwer).
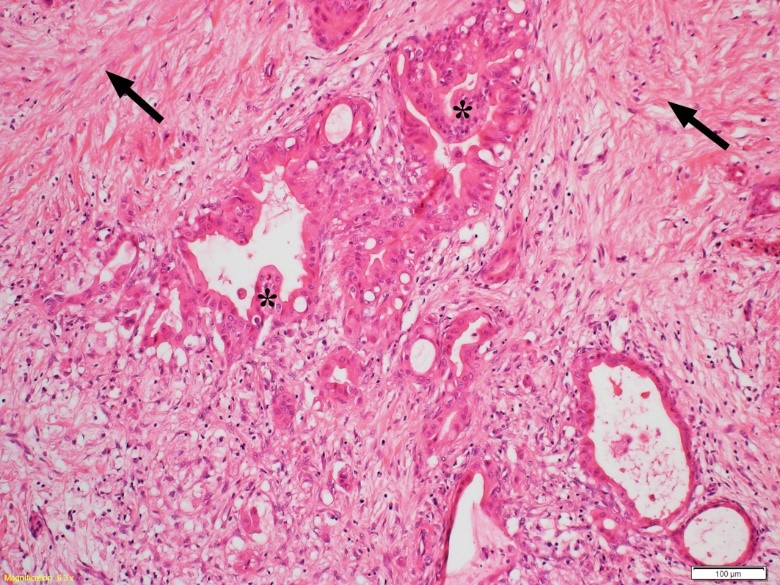
Figure 4. Desmoplasia in a human pancreatic cancer section. A representative photomicrograph of a hematoxylin and eosin stained human pancreatic cancer section showing malignant elements (duct-like and tubular structures- indicated by asterisks) embedded in highly fibrotic stroma (indicated by arrows). Source: Apte et al. 2015 (8). (Reproduced with permission from Elsevier).
Many factors are known to activate PSCs during chronic pancreatitis including the profibrogenic growth factor TGFβ, platelet-derived growth factor (PDGF) (44), nerve growth factor (NGF) (31) and oxidative stress (110). It is now known that PSC-activating factors such as TGFβ, PDGF, oxidant stress and other cytokines are upregulated early during pancreatic injury leading to PSC proliferation and ECM synthesis. As noted earlier, PSCs can be maintained in a perpetually activated state through autocrine effects of endogenous cytokines, leading to pathological fibrosis.
Animal models provide an invaluable tool for understanding the pathogenesis of chronic pancreatitis. Several methods of inducing experimental chronic pancreatitis have been reported in the literature - repeated intravenous injections of caerulein (89) or superoxide dismutase inhibitor (84), instillation of toxins into the pancreatic duct (28, 44); chronic ethanol administration followed by secondary challenge with caerulein (129, 131), cyclosporin (43) or endotoxin (136). Regarding alcoholic chronic pancreatitis, endotoxin is a relevant trigger factor, given that gut permeability is known to be increased by alcohol and elevated serum endotoxin levels have been reported in drinkers (35). Further, endotoxin (lipopolysaccharide, LPS) challenge was shown to enhance fibrosis in Sprague-Dawley rats fed Lieber de Carli alcohol liquid diet (124). Transgenic models of CP include animals overexpressing cytokines or profibrogenic factors such as IL-1β, TGFβ, and heparin‐binding EGF‐like growth factor (HB‐EGF) (15, 75) and WBN–Kob rats (93) and Goto–Kakizaki rats with type 2 diabetes (19). More recently, the effects of smoking on pancreatic fibrosis have been studied, given the close association of drinking, and smoking as lifestyle factors. Lugea and colleagues (73) have shown that smoking significantly worsened tissue injury as well as fibrosis in a rat model of alcoholic pancreatitis. The increased fibrosis could be due to direct activation of PSCs by i) nicotine and NNK via nicotinic acetylcholine receptors (nAChRs) on the cells and/or ii) IL-22 secreted by infiltrating macrophages in response to smoke compounds (via Aryl hydrolase receptors) (154).
V. REVERSAL OF PANCREATIC FIBROSIS IN CHRONIC PANCREATITIS
The study of mechanisms underlying PSC activation and fibrosis can help in developing novel anti-fibrotic treatments. Several antifibrotic strategies have been successfully evaluated to counter CP in experimental animal models including (i) inhibition of profibrogenic growth factors TGFβ and tumor necrosis factor alpha (TNFα) (52, 86, 99, 154); (ii) antioxidants such as vitamin E (40), ellagic acid, a plant polyphenol (125), and salvianolic acid, a herbal medicine (72); (iii) protease inhibitors (39); (iv) modulation of signaling molecules (e.g., troglitazone binding to the peroxisome proliferator receptor gamma, PPARγ) (117); retinoic acid-induced PSC quiescence via suppression of the Wnt–catenin pathway (148); (v) collagen siRNA (55); (vi) an anthraquinone derivative Rhein and a flavonoid, apigenin; (vii) a prostacyclin analogue ONO-1301, which inhibits proinflammatory and profibrogenic cytokine production (91); and (viii) alcohol withdrawal in alcohol induced pancreatitis (134). Amygdalin was shown to inhibit PSC activation and attenuate fibrosis by decreasing production of pro-fibrotic cytokines in a rat CP model induced by injecting dibutyltin dichloride (DBTC) (165). An experimental compound miR-200a inhibited TGF-β1-induced pancreatic stellate cell activation and extracellular matrix formation through inhibition of PTEN /Akt/mTOR pathway (149). In chronic pancreatitis induced by dibutylin dichloride in rats, 3-methyladenine (3-MA), a PI3K inhibitor decreased fibrosis by decreasing autophagy in PSCs (68).
Several plant compounds have shown promising antifibrotic activity in several studies. Curcumin (a polyphenol found in turmeric) was reported to inhibit activation of PSCs through the inhibition of IL1β and TNFα-induced activation of activator protein-1 (AP-1) and mitogen-activated protein (MAP) kinases (ERK, c-Jun N-terminal kinase (JNK), and p38 MAP kinase) (82). Conophylline, a plant alkaloid, decreased fibrosis through the inhibition of ERK1/2 in PSCs (127). In an in vitro study, resveratrol, a natural polyphenol, decreased oxidative stress induced activation and glycolysis in PSCs mediated by ROS/miR-21 (157). Genestein, a natural isoflavone decreased fibrosis in human PSCs transfected with let-7d to express thrombospondin 1, a marker of fibrosis (10). Saikosponin A, the active component of the Chinese medicine chaihu, was shown to decrease PSC activation, viability, proliferation and migration and to promote apoptosis by inhibiting autophagy and the formation of NLRP3 inflammasome via the AMPK/mTOR pathway (24). Similarly, date palm fruit extract decreased fibronectin-1 and αSMA, markers of fibrosis in PSCs activated by TNF-α (1).
Vitamins such as D and its isoforms D2 and D3 are reported to decrease in vitro PSC activation by decreasing IL-6 (139). Furthermore, in vivo studies with the vitamin D ligand calcipotriol have shown significant attenuation of the fibrosis of chronic pancreatitis in mice (114). Since storage of vitamin A is associated with PSC quiescence, administration of vitamin A (retinol) or its metabolites such as retinoic acid, has been assessed in models of chronic pancreatitis. In this regard, Vitamin A-containing liposomes combined with TLR4-silencing shRNA has been reported to inhibit pancreatic fibrosis in mouse models of chronic pancreatitis (166).
Other compounds known to induce quiescence of activated PSCs include melatonin, the anthraquinone derivative rhein (128), bone morphogenic protein (37), troglitazone (a ligand for the peroxisome proliferator activated receptor PPARγ) (117). Recently, kinase inhibitors such as sorafenib, sunitinib, trametinib, and dactolisib have been shown to inhibit PSC proliferation and ECM synthesis (27, 146). Interestingly, trametinib also decreases the expression of two autocrine mediators of PSC activation, IL6 and TGFβ (146). Coenzyme Q10 suppresses PSC activation by inhibiting autophagy through PI3K/ATK/mTOR signaling (155). Inhibition of cyclo-oxygenase by indomethacin, an anti-inflammatory drug also results in decreased activation of PSCs (123).
VI. PANCREATIC CANCER
Pancreatic ductal adenocarcinoma (PDAC) is characterized by extensive stroma/desmoplasia (Figure 5) which constitutes 80 - 90% of the tumor volume (47). This stroma consists of extracellular matrix proteins including collagen type I, fibronectin and laminin, non-collagenous factors such as glycoaminoglycans (e.g., hyaluronan), glycoproteins, and proteoglycans, and several cell types, including stellate cells, endothelial cells, neural elements and immune cells (30).
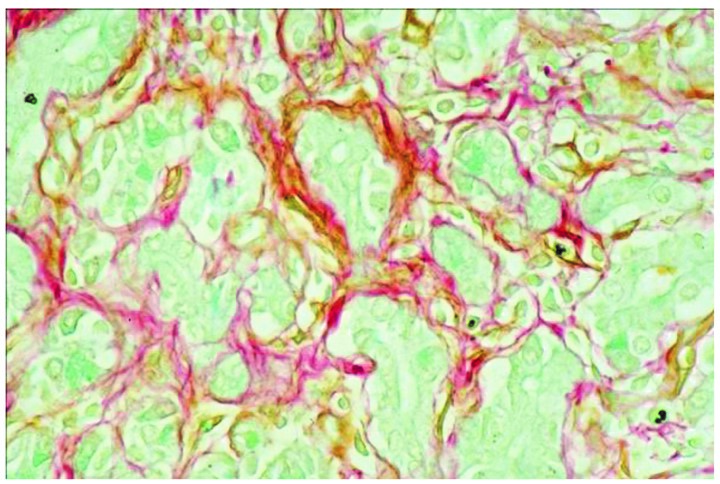
Figure 5. Dual staining for alpha smooth muscle actin (αSMA) and collagen mRNA in a human pancreatic cancer section. Immunostaining for αSMA (brown) combined with in situ hybridization for collagen mRNA (blue) reveals colocalization of the two stains in stromal areas of the section with no staining in tumor cells. This pattern of staining indicates that pancreatic stellate cells are the main source of collagen in pancreatic cancer stroma. Source: Apte et al. 2004 (6). (Reproduced with permission of Wolters Kluwer).
Just as with the fibrosis of chronic pancreatitis, activated PSCs are now also recognised as the primary source of the collagenous stroma in PDAC (6). The detection of activated PSCs expressing periostin (a cell adhesion protein), galectin-1 (a glycan binding protein) and αSMA (an activation marker) in precursor pancreatic intraepithelial neoplasms (PanINs) (Figure 6) and intra-ductal papillary mucinous neoplasms (IPMN) suggests that activation of PSCs is an early event in PDAC (7, 56). Studies have also shown a positive correlation between extent of PSC activation in the stroma and poor clinical outcome (29, 141). It is now known that functionally different subsets of PSCs exist within the stroma of pancreatic cancer. Yuzawa et al. (161) observed the presence of fibroblasts with varying expression of αSMA and PDGFRβ while Ohlund et al. (94) observed that cancer-associated fibroblasts (CAFs) / pancreatic stellate cells in proximity to cancer cells exhibit higher αSMA expression compared to those located at a distance from cancer cells. The authors termed the PSCs close to cancer cells as (myofibroblastic) myPSCs and those at some distance from cancer cells as (inflammatory) iPSCS, based on their cytokine secretion profile.
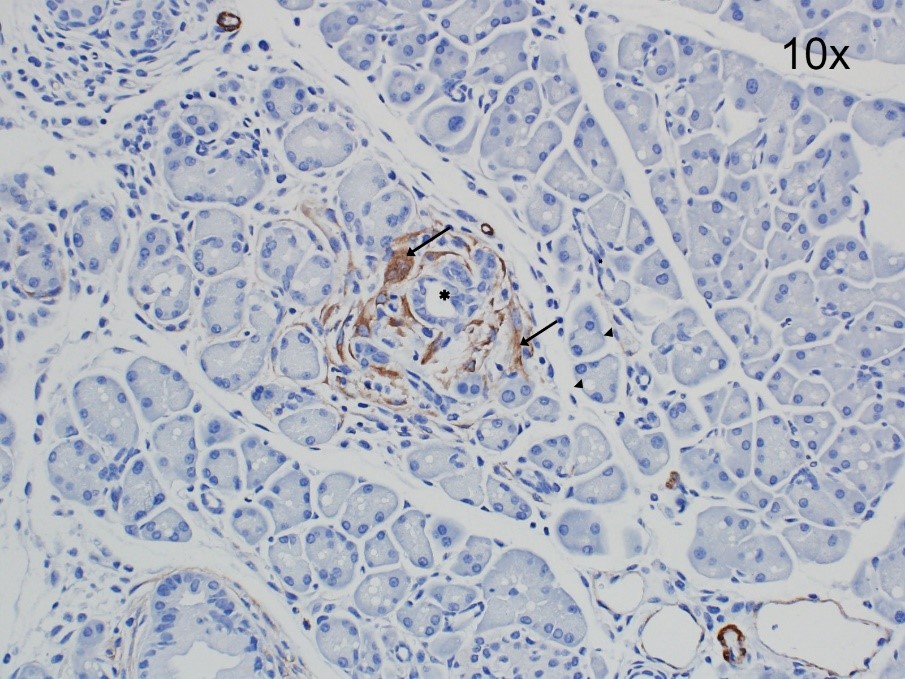
Figure 6. Activated PSCs in early pancreatic intraepithelial neoplasia (PanIN). A section from an 8-week old transgenic KrasG12D P53R172H Cre (KPC) mouse showing staining for the PSC-activation marker alpha smooth muscle actin (αSMA, brown) around PanINs in a section of the pancreas. Magnification 20x.
Studies involving in vitro and in vivo models have established that PSCs interact bidirectionally with cancer cells to facilitate local tumor growth and distant metastasis (8). PSCs also interact with other cells in the stroma including immune cells resulting in a complex cascade of events (Figure 7). PSCs promote cancer cell progression, migration, and cancer cell survival (132, 153) while in turn, cancer cells promote PSC proliferation, migration, and extracellular matrix synthesis (53, 132). Several in vitro studies have shown that cancer cells increase PSC proliferation and ECM synthesis mediated by PDGF, FGF2, and TGFβ1 (12) and also promote the secretion of matrix metalloproteinases by PSCs (97) through ECM metalloproteinase inducer (EMMPRIN) secretion (111) and TGFβ1 signalling (132, 133). Cancer cells can also induce autophagy in PSCs leading to release of alanine which acts as an alternative carbon source for the TCA cycle and lipid synthesis in cancer cells, thus improving cancer cell survival in the nutrient-poor and hypoxic environment of PDAC (51, 119).
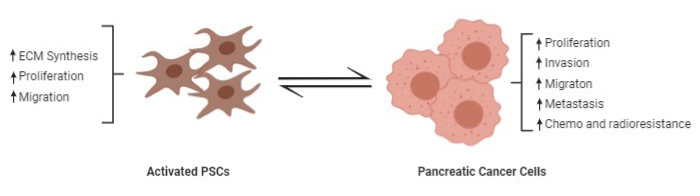
Figure 7. Bi-directional interactions of pancreatic stellate cells with cancer cells. Activated PSCs promote proliferation, invasion, metastasis, survival, and chemoresistance as well as radio-resistance of cancer cells while cancer cells in turn promote ECM synthesis, proliferation, and migration of PSCs.
Epithelial mesenchymal transition (EMT) and stemness, two processes that play an important role in cancer metastasis and recurrence, are known to be induced by PSCs. (45, 61). Studies have shown that stromal signalling is indispensable for cancer progression. Sherman and colleagues have reported that KRas mutation alone is insufficient for pancreatic cancer and that stromal cues, collagen and cytokines derived from cancer associated PSCs, are essential to this process (115). Furthermore, in both subcutaneous and orthotopic xenograft models, co-injection of cancer cells and stromal cells (PSCs) resulted in larger tumors with significant desmoplasia compared to injection of cancer cells alone (53, 104, 132). An intriguing report published some years ago suggested that PSCs may also facilitate seeding of cancer cells at metastatic sites. Using a gender mismatch approach and an orthotopic model of pancreatic cancer, the authors of this study found PSCs from the primary tumor within metastatic nodules at distant sites, indicating that, in addition to cancer cells, PSCs can also disseminate via the circulation (153). Similar observations were made by Suetsugu et al. (121) who injected a mixture of PSCs and pancreatic cancer cells into the spleen and observed that both cell types co-migrated from the spleen to metastatic nodules. In a recent groundbreaking study, Pang et al. (98) have identified the presence of circulating PSCs alongside circulating tumor cells (CTCs) in the blood of orthotopic tumor bearing mice.
In recent years, there has been considerable focus on the role of exosomes (nanosized vesicles secreted by most cell types) in a variety of diseases. These exosomes carry a cargo of proteins, lipids, DNA, mRNA, and microRNA) which can influence the function of cells remote from the source of the exosomes. Regarding pancreatic cancer, Takikawa et al. (126) have demonstrated that exosomes derived from PSCs contained a variety of microRNAs and an abundance of miR-21-5p and miR-451a which mediated PSC-induced proliferation and migration of PSCs. Similarly, exosomes derived from PSCs were found to stimulate activation, proliferation, and migration of PSCs through upregulation of transforming growth factor β1 (TGFβ1) and tumor necrosis factor (TNF).
Several studies have shown that pancreatic desmoplasia can contribute to chemoresistance by affecting the kinetics of chemotherapeutic agents. Hessmann et al. (50) found that cancer associated activated fibroblasts are resistant to gemcitabine and tend to accumulate and rapidly convert gemcitabine into an inactive metabolite 2′,2′-difluorodeoxyuridine thus contributing to decreased efficacy of gemcitabine on tumor cells. Moreover, growing evidence suggests that PSCs may also directly alter the response of cancer cells to chemotherapeutic agents. Autocrine secretion of IL-6 by PSCs in response to stromal-derived factor 1α (SDF-1α), demonstrated a protective effect on cancer cells from the apoptotic effect of gemcitabine (164). Owing to their chemoresistance, post therapy, PSCs can facilitate proliferation of residual cancer stem-cells leading to recurrence (18).
VII. STRATEGIES TO COUNTER STROMAL-TUMOR INTERACTIONS IN PANCREATIC CANCER
As PSC-cancer cell interactions play an important role in the progression of PDAC, several strategies have been developed to counter these interactions. Several chemicals including phytochemicals and hormones have been shown to decrease stromal-tumor interactions in experimental studies. Metformin, an activator of AMP-activated protein kinase reduced the production of fibrogenic cytokines from cancer cells and inhibited PSC activation in co-culture studies and decreased tumor growth and enhanced chemosensitivity to gemcitabine in orthotopic xenograft model (25). ProAgioQ20, which targets an Integrin α(v)β(3) overexpressed in PSCs, caused apoptosis of cancer associated PSCs, reabsorbed collagen, inhibited tumor growth, and drug penetration in subcutaneous, orthotopic xenograft and transgenic KPC model (21). Similarly, inhibition of cyclic AMP-response element binding protein-binding protein (CBP)/β-catenin signaling using ICG-001 resulted in decreased activation of PSCs and PSC-induced migration of PANC-1 cancer cells in a transwell migration assay (23). Curcumin, the active compound of turmeric was shown to inhibit IL-6 in PSCs under hypoxic conditions leading to suppression of EMT and PSC-mediated cancer cell migration in vitro (70). Relaxin-2, an endogenous hormone combined super magnetic iron oxide nanoparticles decreased fibrosis and tumor growth besides potentiating the effects of co-administered gemcitabine in an orthotopic xenograft model (74).
Several pathways are shown to be key regulators of cancer cell-PSC interactions and inhibition of these pathways in animal studies has been reported to decease fibrosis, tumor growth and metastasis. In this regard, Hedgehog pathway is significant as the ligand sonic hedgehog (Shh) is expressed in tumor cells while the receptor, Smoothened (Smo), is located mainly on cancer-associated fibroblasts (140). In animal models, inhibitors of this pathway such as ormeloxifene (59) and IPI-926 (96) decreased desmoplasia and tumor growth and improved chemosensitivity to co-administered gemcitabine. Interestingly, in an orthotopic tumor model where cancer and PSCs are co-injected, a triple combination of CXCR4 antagonist (AMD3100), hedgehog inhibitor (GDC0449) and gemcitabine resulted in complete remission of tumor growth (58).
Similar to the hedgehog pathway, the HGF/c-MET pathway is involved in cancer proliferation, invasion and metastasis. The ligand HGF is produced by PSCs while its receptor, c-MET, is located on cancer cells and endothelial cells (103). In transgenic KPC mice, dual inhibition of both sonic hedgehog and HGF/c-MET pathways were found to sensitize PDAC to gemcitabine (109). In a subcutaneous xenograft mouse model, combination of c-Met inhibitor XL184 with gemcitabine significantly decreased tumor growth compared to individual treatments (67). Xu et al. (151) used a triple therapy approach consisting of HGF and c-Met inhibitors plus gemcitabine in an advanced model of orthotopic pancreatic cancer. Triple therapy not only reduced tumor size, but importantly, eliminated metastasis. Further, the authors reported that the combination therapy was successful in overcoming gemcitabine-stimulated stemness and aggressiveness of cancer cells. These findings corroborate the findings of previous studies that reported that gemcitabine alone increases stemness of cancer cells in PDAC (49, 107).
Extracellular signal-regulated kinases (ERKs) regulate cellular processes such as proliferation, differentiation, transcription and development and this pathway has been shown to be strongly expressed in cancer-associated PSCs. Dual inhibition of ERK1/2 and autophagy in PSCs with S7101 and chloroquine respectively resulted in PSC senescence and suppressed liver metastasis in a splenic pancreatic cancer organoid mouse model (158).
Vitamins inducing PSC quiescence have been successfully demonstrated to improve the outcome of pancreatic cancer in both preclinical and clinical studies. PEGylated gold nanoparticles containing all trans retinoic acid (ATRA,) combined with siRNA against heat shock protein (HSP-47, known to activate PSCs) was used to induce PSC quiescence and inhibited ECM hyperplasia, thereby promoting drug delivery to pancreatic tumors in an orthotopic model developed by co-inoculation of cancer cells and PSCs (46). Similarly, the vitamin D analogue calcipotriol, combined with gemcitabine, induced quiescence of activated PSCs and decreased fibrosis in KPC mice (114). Based on this study, a vitamin D analogue paricalcitol, in combination with gemcitabine, and nab-paclitaxel is currently being evaluated in a clinical trial (NCT03520790) (100).
As epigenetic modifications also play an important role in PDAC, some studies have targeted epigenetic pathways to reprogram the tumor microenvironment in a bid to improve outcomes (90, 156). Common epigenetic targets relevant to PDAC include DNA methylation, histone modifications and bromodomain and extra-terminal domain (BET) family proteins. A DNA methyl transferase inhibitor, 5-Azacitidine, in combination with abraxane and gemcitabine is currently being tested in a Phase II clinical trial in resected PDAC patients (NCT01845805). Inhibiting epigenetic bromodomain and the extraterminal domain (BET) family of proteins using iBET151, and SB 203580, a MAPK inhibitor, decreased YAP-1 levels resulting in decreased PSC activation (51).
Myeloid derived suppressor cells (MDSC) help to maintain an immunosuppressive environment in PDAC by eliminating effector T cells (36). Therefore, therapies that boost effector T cell infiltration into tumor are gaining attention. The desmoplastic reaction in PDAC is shown to sequester cytotoxic CD8+ T cells and hinder drug penetration. In transgenic spontaneous pancreatic cancer bearing KC mice (KrasG12D,Cre) challenged with caerulein, an inhibitor of rho/ MRTF pathway, CCG-222740 not only decreased PSC activation but also decreased infiltration of macrophages, CD4+ T cells and B cells suggesting inhibition of PSC activation improves local immunity (66). Similarly, inhibiting stroma through an acidic-pH-responsive-nanoparticle cluster containing TGF-β inhibitor (LY2157299) and PD-L1 (siRNA) improved local tumor immunity by increasing CD8+ T cell infiltration in a subcutaneous xenograft orthotopic model (143). Tumor associated macrophages (TAM), which are derived from MDSCs, are shown to rapidly inactivate gemcitabine and depletion of TAM using clodronate resulted in increased concentration of active gemcitabine in desmoplastic tumors of KPC mice compared to either PanINs or normal pancreas (17).
VIII. CONCLUSION
PSCs play manifold roles in both health and disease. In the healthy pancreas they are responsible for maintaining ECM turnover and may also have additional roles in innate immunity and CCK-mediated exocrine secretion. In diseased conditions, PSCs are activated by a variety of factors known to be upregulated in the injured pancreas. The last two decades have seen an explosion of findings regarding the interaction of PSCs with other cells in the pancreas, in health and in disease. These findings have vastly improved our understanding of the physiology and pathology of pancreatic disease, raising hopes for innovative new treatments. Once activated, they become the primary contributors to the pathological fibrosis of both pancreatic inflammation and PDAC and impact on the function of beta cells of islets (or some reference to endocrine pancreas). Research efforts are now directed towards understanding the mechanisms underlying PSC-mediated fibrogenesis to develop novel therapies to treat fibrotic diseases of the pancreas.
IX. REFERENCES
- Al Alawi R, Alhamdani MSS, Hoheisel JD, and Baqi Y. Antifibrotic and tumor microenvironment modulating effect of date palm fruit (Phoenix dactylifera L.) extracts in pancreatic cancer. Biomed Pharmacother 121: 109522, 2020. PMID: 31675539.
- Andoh A, Takaya H, Saotome T, Shimada M, Hata K, Araki Y, Nakamura F, Shintani Y, Fujiyama Y, and Bamba T. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 119: 211-219, 2000. PMID: 10889171.
- Apte M, Pirola R, and Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal 15: 2711-2722, 2011. PMID: 21728885.
- Apte M, Pirola RC, and Wilson JS. Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr Opin Gastroenterol 31: 416-423, 2015. PMID: 26125317.
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, and Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43: 128-133, 1998. PMID: 9771417.
- strong>Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, and Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29: 179-187, 2004. PMID: 15367883.
- Apte MV, Wilson JS, Lugea A, and Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144: 1210-1219, 2013. PMID: 23622130.
- Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, and Wilson JS. Pancreatic cancer: The microenvironment needs attention too! Pancreatology 15: S32-38, 2015. PMID: 25845856.
- Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr., Sucov HM, and Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 49: 998-1011, 2009. PMID: 19085956.
- Asama H, Suzuki R, Hikichi T, Takagi T, Masamune A, and Ohira H. MicroRNA let-7d targets thrombospondin-1 and inhibits the activation of human pancreatic stellate cells. Pancreatology 19: 196-203, 2019. PMID: 30393009.
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, and Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115: 421-432, 1998. PMID: 9679048.
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, and Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128: 907-921, 2005. PMID: 15825074.
- Ben-Harosh Y, Anosov M, Salem H, Yatchenko Y, and Birk R. Pancreatic stellate cell activation is regulated by fatty acids and ER stress. Exp Cell Res 359: 76-85, 2017. PMID: 28827060.
- Berna MJ, Seiz O, Nast JF, Benten D, Blaker M, Koch J, Lohse AW, and Pace A. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem 285: 38905-38914, 2010. PMID: 20843811.
- Blaine SA, Ray KC, Branch KM, Robinson PS, Whitehead RH, and Means AL. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol 297: G434-441, 2009. PMID: 19608732.
- Brizzi MF, Tarone G, and Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol 24: 645-651, 2012. PMID: 22898530.
- Buchholz SM, Goetze RG, Singh SK, Ammer-Herrmenau C, Richards FM, Jodrell DI, Buchholz M, Michl P, Ellenrieder V, Hessmann E, and Neesse A. Depletion of Macrophages Improves Therapeutic Response to Gemcitabine in Murine Pancreas Cancer. Cancers (Basel) 12, 2020. PMID: 32698524.
- Cabrera MC, Tilahun E, Nakles R, Diaz-Cruz ES, Charabaty A, Suy S, Jackson P, Ley L, Slack R, Jha R, Collins SP, Haddad N, Kallakury BV, Schroeder T, Pishvaian MJ, and Furth PA. Human Pancreatic Cancer-Associated Stellate Cells Remain Activated after in vivo Chemoradiation. Front Oncol 4: 102, 2014. PMID: 24847445.
- Calderari S, Irminger JC, Giroix MH, Ehses JA, Gangnerau MN, Coulaud J, Rickenbach K, Gauguier D, Halban P, Serradas P, and Homo-Delarche F. Regenerating 1 and 3b gene expression in the pancreas of type 2 diabetic Goto-Kakizaki (GK) rats. PLoS One 9: e90045, 2014. PMID: 24587207.
- Casini A, Galli A, Pignalosa P, Frulloni L, Grappone C, Milani S, Pederzoli P, Cavallini G, and Surrenti C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 192: 81-89, 2000. PMID: 10951404.
- Chakra Turaga R, Sharma M, Mishra F, Krasinskas A, Yuan Y, Yang JJ, Wang S, Liu C, Li S, and Liu ZR. Modulation of Cancer-Associated Fibrotic Stroma by An Integrin alphavbeta3 Targeting Protein for Pancreatic Cancer Treatment. Cell Mol Gastroenterol Hepatol, 2020. PMID: 32810598.
- Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, and Brigstock DR. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell Commun Signal 8: 147-156, 2014. PMID: 24464300.
- Che M, Kweon SM, Teo JL, Yuan YC, Melstrom LG, Waldron RT, Lugea A, Urrutia RA, Pandol SJ, and Lai KKY. Targeting the CBP/beta-Catenin Interaction to Suppress Activation of Cancer-Promoting Pancreatic Stellate Cells. Cancers (Basel) 12, 2020. PMID: 32516943.
- Cui L, Li C, Zhuo Y, Yang L, Cui N, Li Y, and Zhang S. Saikosaponin A inhibits the activation of pancreatic stellate cells by suppressing autophagy and the NLRP3 inflammasome via the AMPK/mTOR pathway. Biomed Pharmacother 128: 110216, 2020. PMID: 32497863.
- Duan W, Chen K, Jiang Z, Chen X, Sun L, Li J, Lei J, Xu Q, Ma J, Li X, Han L, Wang Z, Wu Z, Wang F, Wu E, Ma Q, and Ma Z. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett 385: 225-233, 2017. PMID: 27773749.
- Elsasser HP, Adler G, and Kern HF. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas 1: 421-429, 1986. PMID: 2436216.
- Elsner A, Lange F, Fitzner B, Heuschkel M, Krause BJ, and Jaster R. Distinct antifibrogenic effects of erlotinib, sunitinib and sorafenib on rat pancreatic stellate cells. World J Gastroenterol 20: 7914-7925, 2014. PMID: 24976727.
- Emmrich F, Stahl HD, and Altrichter S. [Leipzig Center for Therapy Studies--a cooperative structure for realizing clinical studies]. Z Rheumatol 59: 57-60, 2000. PMID: 10769427.
- Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, and Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 6: 1155-1161, 2008. PMID: 28639493.
- Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, and Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 18: 4266-4276, 2012. PMID: 22896693.
- Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, and Buchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 230: 615-624, 1999. PMID: 10561084.
- Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, and Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 141: 1486-1497, 1497 e1481-1414, 2011. PMID: 21704588.
- Frossard JL, Steer ML, and Pastor CM. Acute pancreatitis. Lancet 371: 143-152, 2008. PMID: 18191686.
- Fu Y, Liu S, Zeng S, and Shen H. The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol Cancer 17: 62, 2018. PMID: 29458370.
- Fukui H, Brauner B, Bode JC, and Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12: 162-169, 1991. PMID: 2050995.
- Gabrilovich DI, Ostrand-Rosenberg S, and Bronte V. Coordinated regulation of myeloid cells by tumors. Nat Rev Immunol 12: 253-268, 2012. PMID: 22437938.
- Gao X, Cao Y, Staloch DA, Gonzales MA, Aronson JF, Chao C, Hellmich MR, and Ko TC. Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis. PLoS One 9: e89114, 2014. PMID: 24586530.
- Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, Ban Y, Lavania S, Dawra R, Banerjee S, Vickers S, Merchant NB, Chen SX, Gilboa E, Ramakrishnan S, Saluja A, and Dudeja V. NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12. Gastroenterology 155: 880-891 e888, 2018. PMID: 29909021.
- Gibo J, Ito T, Kawabe K, Hisano T, Inoue M, Fujimori N, Oono T, Arita Y, and Nawata H. Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity. Lab Invest 85: 75-89, 2005. PMID: 15531908.
- Gomez JA, Molero X, Vaquero E, Alonso A, Salas A, and Malagelada JR. Vitamin E attenuates biochemical and morphological features associated with development of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 287: G162-169, 2004. PMID: 15001429.
- Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, Cruz-Monserrate Z, Fuentes-Mattei E, Deng D, Hwang RF, Wang H, Ivan C, Garza RJ, Cohen E, Gao H, Armaiz-Pena GN, Del CM-BP, Philip B, Rashed MH, Aslan B, Erdogan MA, Gutierrez-Puente Y, Ozpolat B, Reuben JM, Sood AK, Logsdon C, and Lopez-Berestein G. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther 13: 2583-2594, 2014. PMID: 25193509.
- Gryshchenko O, Gerasimenko JV, Gerasimenko OV, and Petersen OH. Ca(2+) signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca(2+) channel blockade. J Physiol 594: 281-293, 2016. PMID: 26442817.
- Gukovsky I, Lugea A, Shahsahebi M, Cheng JH, Hong PP, Jung YJ, Deng QG, French BA, Lungo W, French SW, Tsukamoto H, and Pandol SJ. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 294: G68-79, 2008. PMID: 17884979.
- Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, and Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 155: 1087-1095, 1999. PMID: 10514391.
- Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, Hamada H, Kobune M, Satoh K, and Shimosegawa T. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun 421: 349-354, 2012. PMID: 22510406.
- Han X, Li Y, Xu Y, Zhao X, Zhang Y, Yang X, Wang Y, Zhao R, Anderson GJ, Zhao Y, and Nie G. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumor microenvironment-activated nanosystem. Nat Commun 9: 3390, 2018. PMID: 30139933.
- Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, Innocenti P, Giese T, Giese N, Buchler MW, and Friess H. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg 28: 818-825, 2004. PMID: 15457365.
- Hennigs JK, Seiz O, Spiro J, Berna MJ, Baumann HJ, Klose H, and Pace A. Molecular basis of P2-receptor-mediated calcium signaling in activated pancreatic stellate cells. Pancreas 40: 740-746, 2011. PMID: 21654543.
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, and Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1: 313-323, 2007. PMID: 18371365.
- Hessmann E, Patzak MS, Klein L, Chen N, Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, Jodrell DI, Verbeke C, Li X, Heuchel R, Lohr JM, Johnsen SA, Gress TM, Ellenrieder V, and Neesse A. Fibroblast drug scavenging increases intratumoral gemcitabine accumulation in murine pancreas cancer. Gut 67: 497-507, 2018. PMID: 28077438.
- Hu C, Yang J, Su H-Y, Waldron RT, Zhi M, Li L, Xia Q, Pandol SJ, and Lugea A. Yes-Associated Protein 1 Plays Major Roles in Pancreatic Stellate Cell Activation and Fibroinflammatory Responses. Frontiers in Physiology 10, 2019. PMID: 31849712.
- Hughes CB, Gaber LW, Mohey el-Din AB, Grewal HP, Kotb M, Mann L, and Gaber AO. Inhibition of TNF alpha improves survival in an experimental model of acute pancreatitis. Am Surg 62: 8-13, 1996. PMID: 8540653.
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, and Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68: 918-926, 2008. PMID: 18245495.
- Ikejiri N. The vitamin A-storing cells in the human and rat pancreas. Kurume Med J 37: 67-81, 1990. PMID: 2255178.
- Ishiwatari H, Sato Y, Murase K, Yoneda A, Fujita R, Nishita H, Birukawa NK, Hayashi T, Sato T, Miyanishi K, Takimoto R, Kobune M, Ota S, Kimura Y, Hirata K, Kato J, and Niitsu Y. Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut 62: 1328-1339, 2013. PMID: 23172890.
- Kakizaki Y, Makino N, Tozawa T, Honda T, Matsuda A, Ikeda Y, Ito M, Saito Y, Kimura W, and Ueno Y. Stromal Fibrosis and Expression of Matricellular Proteins Correlate With Histological Grade of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 45: 1145-1152, 2016. PMID: 26967452.
- Karger A, Fitzner B, Brock P, Sparmann G, Emmrich J, Liebe S, and Jaster R. Molecular insights into connective tissue growth factor action in rat pancreatic stellate cells. Cell Signal 20: 1865-1872, 2008. PMID: 18639630.
- Khan MA, Srivastava SK, Zubair H, Patel GK, Arora S, Khushman M, Carter JE, Gorman GS, Singh S, and Singh AP. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J Biol Chem 295: 8413-8424, 2020. PMID: 32358063.
- Khan S, Ebeling MC, Chauhan N, Thompson PA, Gara RK, Ganju A, Yallapu MM, Behrman SW, Zhao H, Zafar N, Singh MM, Jaggi M, and Chauhan SC. Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Cancer Res 75: 2292-2304, 2015. PMID: 25840985.
- Kikuta K, Masamune A, Hamada S, Takikawa T, Nakano E, and Shimosegawa T. Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun 433: 292-297, 2013. PMID: 23500461.
- Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, Satoh K, Egawa S, Unno M, and Shimosegawa T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun 403: 380-384, 2010. PMID: 21081113.
- Kloppel G. Pathology of chronic pancreatitis and pancreatic pain. Acta Chir Scand 156: 261-265, 1990. PMID: 2349844.
- Komar HM, Serpa G, Kerscher C, Schwoegl E, Mace TA, Jin M, Yang MC, Chen CS, Bloomston M, Ostrowski MC, Hart PA, Conwell DL, and Lesinski GB. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep 7: 1787, 2017. PMID: 28496202.
- Kordes C, Sawitza I, and Haussinger D. Hepatic and pancreatic stellate cells in focus. Biol Chem 390: 1003-1012, 2009. PMID: 19642878.
- Kumar K, DeCant BT, Grippo PJ, Hwang RF, Bentrem DJ, Ebine K, and Munshi HG. BET inhibitors block pancreatic stellate cell collagen I production and attenuate fibrosis in vivo. JCI Insight 2: e88032, 2017. PMID: 28194432.
- Leal AS, Misek SA, Lisabeth EM, Neubig RR, and Liby KT. The Rho/MRTF pathway inhibitor CCG-222740 reduces stellate cell activation and modulates immune cell populations in Kras(G12D); Pdx1-Cre (KC) mice. Sci Rep 9: 7072, 2019. PMID: 31068602.
- Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, and Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141: 2218-2227 e2215, 2011. PMID: 21864475.
- Li CX, Cui LH, Zhuo YZ, Hu JG, Cui NQ, and Zhang SK. Inhibiting autophagy promotes collagen degradation by regulating matrix metalloproteinases in pancreatic stellate cells. Life Sci 208: 276-283, 2018. PMID: 30056017.
- Li N, Li Y, Li Z, Huang C, Yang Y, Lang M, Cao J, Jiang W, Xu Y, Dong J, and Ren H. Hypoxia Inducible Factor 1 (HIF-1) Recruits Macrophage to Activate Pancreatic Stellate Cells in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci 17, 2016. PMID: 27271610.
- Li W, Sun L, Lei J, Wu Z, Ma Q, and Wang Z. Curcumin inhibits pancreatic cancer cell invasion and EMT by interfering with tumorstromal crosstalk under hypoxic conditions via the IL6/ERK/NFkappaB axis. Oncol Rep 44: 382-392, 2020. PMID: 32377752.
- Liu Y, Li F, Gao F, Xing L, Qin P, Liang X, Zhang J, Qiao X, Lin L, Zhao Q, and Du L. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumor Biol 37: 15283-15291, 2016. PMID: 27696296.
- Lu XL, Dong XY, Fu YB, Cai JT, Du Q, Si JM, and Mao JS. Protective effect of salvianolic acid B on chronic pancreatitis induced by trinitrobenzene sulfonic acid solution in rats. Pancreas 38: 71-77, 2009. PMID: 18766118.
- Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu C, French SW, Wilson JS, Apte MV, Waldron RT, and Pandol SJ. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology 153: 1674-1686, 2017. PMID: 28847752.
- Mardhian DF, Storm G, Bansal R, and Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J Control Release 290: 1-10, 2018. PMID: 30287265.
- Marrache F, Tu SP, Bhagat G, Pendyala S, Osterreicher CH, Gordon S, Ramanathan V, Penz-Osterreicher M, Betz KS, Song Z, and Wang TC. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology 135: 1277-1287, 2008. PMID: 18789941.
- Masamune A, Kikuta K, Satoh M, Satoh K, and Shimosegawa T. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol 140: 1292-1302, 2003. PMID: 14581180.
- Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, and Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 295: G709-717, 2008. PMID: 18669622.
- Masamune A, Kikuta K, Watanabe T, Satoh K, Satoh A, and Shimosegawa T. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol 43: 352-362, 2008. PMID: 18592153.
- Masamune A, Nakano E, Hamada S, Takikawa T, Yoshida N, and Shimosegawa T. Alteration of the microRNA expression profile during the activation of pancreatic stellate cells. Scand J Gastroenterol 49: 323-331, 2014. PMID: 24404812.
- Masamune A, Satoh A, Watanabe T, Kikuta K, Satoh M, Suzuki N, Satoh K, and Shimosegawa T. Effects of ethanol and its metabolites on human pancreatic stellate cells. Dig Dis Sci 55: 204-211, 2010. PMID: 19165599.
- Masamune A and Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol 44: 249-260, 2009. PMID: 19271115.
- Masamune A, Suzuki N, Kikuta K, Satoh M, Satoh K, and Shimosegawa T. Curcumin blocks activation of pancreatic stellate cells. J Cell Biochem 97: 1080-1093, 2006. PMID: 16294327.
- Mato E, Lucas M, Petriz J, Gomis R, and Novials A. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J 421: 181-191, 2009. PMID: 19379129.
- Matsumura N, Ochi K, Ichimura M, Mizushima T, Harada H, and Harada M. Study on free radicals and pancreatic fibrosis--pancreatic fibrosis induced by repeated injections of superoxide dismutase inhibitor. Pancreas 22: 53-57, 2001. PMID: 11138971.
- McCarroll JA, Phillips PA, Kumar RK, Park S, Pirola RC, Wilson JS, and Apte MV. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol 67: 1215-1225, 2004. PMID: 15006556.
- Menke A, Yamaguchi H, Gress TM, and Adler G. Extracellular matrix is reduced by inhibition of transforming growth factor beta1 in pancreatitis in the rat. Gastroenterology 113: 295-303, 1997. PMID: 9207290.
- Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, and Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 50: 535-541, 2002. PMID: 11889076.
- Morishita K, Shimizu K, Haruta I, Kawamura S, Kobayashi M, and Shiratori K. Engulfment of gram-positive bacteria by pancreatic stellate cells in pancreatic fibrosis. Pancreas 39: 1002-1007, 2010. PMID: 204314020.
- Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, and Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci 45: 665-674, 2000. PMID: 10759232.
- Nguyen AH, Elliott IA, Wu N, Matsumura C, Vogelauer M, Attar N, Dann A, Ghukasyan R, Toste PA, Patel SG, Williams JL, Li L, Dawson DW, Radu C, Kurdistani SK, and Donahue TR. Histone deacetylase inhibitors provoke a tumor supportive phenotype in pancreatic cancer associated fibroblasts. Oncotarget 8: 19074-19088, 2017. PMID: 27894105.
- Niina Y, Ito T, Oono T, Nakamura T, Fujimori N, Igarashi H, Sakai Y, and Takayanagi R. A sustained prostacyclin analog, ONO-1301, attenuates pancreatic fibrosis in experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Pancreatology 14: 201-210, 2014. PMID: 24854616.
- Nomiyama Y, Tashiro M, Yamaguchi T, Watanabe S, Taguchi M, Asaumi H, Nakamura H, and Otsuki M. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas 34: 364-372, 2007. PMID: 17414061.
- Ohashi K, Kim JH, Hara H, Aso R, Akimoto T, and Nakama K. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol 6: 231-247, 1990. PMID: 1698893.
- Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio, II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, and Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214: 579-596, 2017. PMID: 28232471.
- Ohnishi H, Miyata T, Yasuda H, Satoh Y, Hanatsuka K, Kita H, Ohashi A, Tamada K, Makita N, Iiri T, Ueda N, Mashima H, and Sugano K. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem 279: 8873-8878, 2004. PMID: 14688282.
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, and Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457-1461, 2009. PMID: 19460966.
- Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, and Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol 27 Suppl 2: 127-134, 2012. PMID: 22320930.
- Pang T, Xu Z, Pothula S, Yang MH, Becker T, Goldstein D, Heeschen C, Pirola R, Wilson J, and Apte MV. 517-World-First Identification of Circulating Pancreatic Stellate Cells in Metastatic Pancreatic Cancer. Gastroenterology 154: S-114, 2018.
- Pereda J, Sabater L, Cassinello N, Gomez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J, Cerda M, Lledo S, Vina J, and Sastre J. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg 240: 108-116, 2004. PMID: 15213626.
- Perez K. Vitamin D Receptor Agonist Paricalcitol Plus Gemcitabine and Nab-paclitaxel in Patients With Metastatic Pancreatic Cancer. Identifier NCT03520790, edited by https://clinicaltrials.gov/ct2/show/NCT03520790, 2018.
- Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, and Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 52: 275-282, 2003. PMID: 12524413.
- Phillips PA, Yang L, Shulkes A, Vonlaufen A, Poljak A, Bustamante S, Warren A, Xu Z, Guilhaus M, Pirola R, Apte MV, and Wilson JS. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A 107: 17397-17402, 2010. PMID: 20852067.
- Pothula SP, Xu Z, Goldstein D, Biankin AV, Pirola RC, Wilson JS, and Apte MV. Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer. Br J Cancer 114: 269-280, 2016. PMID: 26766740.
- Pothula SP, Xu Z, Goldstein D, Merrett N, Pirola RC, Wilson JS, and Apte MV. Targeting the HGF/c-MET pathway: stromal remodelling in pancreatic cancer. Oncotarget 8: 76722-76739, 2017. PMID: 29100344.
- Provenzano PP and Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 108: 1-8, 2013. PMID: 23299539.
- Qian D, Lu Z, Xu Q, Wu P, Tian L, Zhao L, Cai B, Yin J, Wu Y, Staveley-O'Carroll KF, Jiang K, Miao Y, and Li G. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett 397: 43-51, 2017. PMID: 28336327.
- Quint K, Tonigold M, Di Fazio P, Montalbano R, Lingelbach S, Ruckert F, Alinger B, Ocker M, and Neureiter D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int J Oncol 41: 2093-2102, 2012. PMID: 23026911.
- Riopel MM, Li J, Liu S, Leask A, and Wang R. beta1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab Invest 93: 31-40, 2013. PMID: 23069938.
- Rucki AA, Xiao Q, Muth S, Chen J, Che X, Kleponis J, Sharma R, Anders RA, Jaffee EM, and Zheng L. Dual Inhibition of Hedgehog and c-Met Pathways for Pancreatic Cancer Treatment. Mol Cancer Ther 16: 2399-2409, 2017. PMID: 28864680.
- Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grunert A, and Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 281: C532-543, 2001. PMID: 11443052.
- Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, Moller P, Gschwend JE, Seufferlein T, Gress T, Adler G, and Bachem MG. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci 120: 512-519, 2007. PMID: 17227797.
- Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, and Iredale JP. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160: 1787-1798, 2002. PMID: 12000730.
- Shen J, Wan R, Hu G, Yang L, Xiong J, Wang F, Shen J, He S, Guo X, Ni J, Guo C, and Wang X. miR-15b and miR-16 induce the apoptosis of rat activated pancreatic stellate cells by targeting Bcl-2 in vitro. Pancreatology 12: 91-99, 2012. PMID: 22487517.
- Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O'Dwyer PJ, Liddle C, Tuveson DA, Downes M, and Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159: 80-93, 2014. PMID: 25259922.
- Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, Leblanc M, Collisson EA, Asara JM, Kimmelman AC, Downes M, and Evans RM. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci U S A 114: 1129-1134, 2017. PMID: 28096419.
- Shimizu K, Kobayashi M, Tahara J, and Shiratori K. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology 128: 2105-2118, 2005. PMID: 15940641.
- Shimizu K, Shiratori K, Kobayashi M, and Kawamata H. Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPARgamma-independent mechanism. Pancreas 29: 67-74, 2004. PMID: 15211114.
- Sicchieri RD, da Silveira WA, Mandarano LR, de Oliveira TM, Carrara HH, Muglia VF, de Andrade JM, and Tiezzi DG. ABCG2 is a potential marker of tumor-initiating cells in breast cancer. Tumor Biol 36: 9233-9243, 2015. PMID: 26091795.
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, and Kimmelman AC. Pancreatic stellate cells support tumor metabolism through autophagic alanine secretion. Nature 536: 479-483, 2016. PMID: 27509858.
- Sparmann G, Kruse ML, Hofmeister-Mielke N, Koczan D, Jaster R, Liebe S, Wolff D, and Emmrich J. Bone marrow-derived pancreatic stellate cells in rats. Cell Res 20: 288-298, 2010. PMID: 20101265.
- Suetsugu A, Snyder CS, Moriwaki H, Saji S, Bouvet M, and Hoffman RM. Imaging the Interaction of Pancreatic Cancer and Stellate Cells in the Tumor Microenvironment during Metastasis. Anticancer Res 35: 2545-2551, 2015. PMID: 25964528.
- Sugimoto R, Enjoji M, Kohjima M, Tsuruta S, Fukushima M, Iwao M, Sonta T, Kotoh K, Inoguchi T, and Nakamuta M. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int 25: 1018-1026, 2005. PMID: 16162162.
- Sun L, Chen K, Jiang Z, Chen X, Ma J, Ma Q, and Duan W. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol Rep 39: 2243-2251, 2018. PMID: 29565462.
- Sun L, Xiu M, Wang S, Brigstock DR, Li H, Qu L, and Gao R. Lipopolysaccharide enhances TGF-beta1 signalling pathway and rat pancreatic fibrosis. J Cell Mol Med 22: 2346-2356, 2018. PMID: 29424488.
- Suzuki N, Masamune A, Kikuta K, Watanabe T, Satoh K, and Shimosegawa T. Ellagic acid inhibits pancreatic fibrosis in male Wistar Bonn/Kobori rats. Dig Dis Sci 54: 802-810, 2009. PMID: 18651219.
- Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, and Shimosegawa T. Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas 46: 19-27, 2017. PMID: 27841793.
- Tezuka T, Ota A, Karnan S, Matsuura K, Yokoo K, Hosokawa Y, Vigetti D, Passi A, Hatano S, Umezawa K, and Watanabe H. The plant alkaloid conophylline inhibits matrix formation of fibroblasts. J Biol Chem 293: 20214-20226, 2018. PMID: 30377255.
- Tsang SW, Zhang H, Lin C, Xiao H, Wong M, Shang H, Yang ZJ, Lu A, Yung KK, and Bian Z. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLoS One 8: e82201, 2013. PMID: 24312641.
- Tsukamoto H, Towner SJ, Yu GS, and French SW. Potentiation of ethanol-induced pancreatic injury by dietary fat. Induction of chronic pancreatitis by alcohol in rats. Am J Pathol 131: 246-257, 1988. PMID: 3358454.
- Uden S, Bilton D, Nathan L, Hunt LP, Main C, and Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther 4: 357-371, 1990. PMID: 2103755.
- Uesugi T, Froh M, Gabele E, Isayama F, Bradford BU, Ikai I, Yamaoka Y, and Arteel GE. Contribution of angiotensin II to alcohol-induced pancreatic fibrosis in rats. J Pharmacol Exp Ther 311: 921-928, 2004. PMID: 15316086.
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, and Apte MV. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68: 2085-2093, 2008. PMID: 18381413.
- Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, and Apte MV. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res 68: 7707-7710, 2008. PMID: 18829522.
- Vonlaufen A, Phillips PA, Xu Z, Zhang X, Yang L, Pirola RC, Wilson JS, and Apte MV. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 60: 238-246, 2011. PMID: 20870739.
- Vonlaufen A, Phillips PA, Yang L, Xu Z, Fiala-Beer E, Zhang X, Pirola RC, Wilson JS, and Apte MV. Isolation of quiescent human pancreatic stellate cells: a promising in vitro tool for studies of human pancreatic stellate cell biology. Pancreatology 10: 434-443, 2010. PMID: 20733342.
- Vonlaufen A, Wilson JS, Pirola RC, and Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health 30: 48-54, 2007. PMID: 17718401.
- Vonlaufen A, Xu Z, Daniel B, Kumar RK, Pirola R, Wilson J, and Apte MV. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 133: 1293-1303, 2007. PMID: 17919500.
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol 66: 303-353, 1980. PMID: 6993411.
- Wallbaum P, Rohde S, Ehlers L, Lange F, Hohn A, Bergner C, Schwarzenbock SM, Krause BJ, and Jaster R. Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J Gastroenterol 24: 170-178, 2018. PMID: 29375203.
- Walter K, Omura N, Hong SM, Griffith M, Vincent A, Borges M, and Goggins M. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res 16: 1781-1789, 2010. PMID: 20215540.
- Wang LM, Silva MA, D'Costa Z, Bockelmann R, Soonawalla Z, Liu S, O'Neill E, Mukherjee S, McKenna WG, Muschel R, and Fokas E. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 7: 4183-4194, 2016. PMID: 26716653.
- Wang T, Wang Q-q, Pan G-x, Jia G-r, Li X, Wang C, Zhang L-m, and Zuo C-j. ASIC1a involves acidic microenvironment-induced activation and autophagy of pancreatic stellate cells. RSC Advances 8: 30950-30956, 2018.
- Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J, Li H, He X, Cao J, and Wang J. Co-inhibition of the TGF-beta pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci 8: 5121-5132, 2020. PMID: 32820750.
- Watari N, Hotta Y, and Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn 58: 837-858, 1982. PMID: 7122019.
- Wilson JS, Pirola RC, and Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol 5: 52, 2014. PMID: 24592240.
- Witteck L and Jaster R. Trametinib and dactolisib but not regorafenib exert antiproliferative effects on rat pancreatic stellate cells. Hepatobiliary Pancreat Dis Int 14: 642-650, 2015. PMID: 26663013.
- Won JH, Zhang Y, Ji B, Logsdon CD, and Yule DI. Phenotypic changes in mouse pancreatic stellate cell Ca2+ signaling events following activation in culture and in a disease model of pancreatitis. Mol Biol Cell 22: 421-436, 2011. PMID: 21148289.
- Xiao W, Jiang W, Shen J, Yin G, Fan Y, Wu D, Qiu L, Yu G, Xing M, Hu G, Wang X, and Wan R. Retinoic Acid Ameliorates Pancreatic Fibrosis and Inhibits the Activation of Pancreatic Stellate Cells in Mice with Experimental Chronic Pancreatitis via Suppressing the Wnt/beta-Catenin Signaling Pathway. PLoS One 10: e0141462, 2015. PMID: 26556479.
- Xu M, Wang G, Zhou H, Cai J, Li P, Zhou M, Lu Y, Jiang X, Huang H, Zhang Y, and Gong A. TGF-beta1-miR-200a-PTEN induces epithelial-mesenchymal transition and fibrosis of pancreatic stellate cells. Mol Cell Biochem 431: 161-168, 2017. PMID: 28281184.
- Xu W, Li W, Wang Y, Zha M, Yao H, Jones PM, and Sun Z. Regenerating islet-derived protein 1 inhibits the activation of islet stellate cells isolated from diabetic mice. Oncotarget 6: 37054-37065, 2015. PMID: 26496027.
- Xu Z, Pang TCY, Liu AC, Pothula SP, Mekapogu AR, Perera CJ, Murakami T, Goldstein D, Pirola RC, Wilson JS, and Apte MV. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: a key element of treatment that limits primary tumor growth and eliminates metastasis. Br J Cancer 122: 1486-1495, 2020. PMID: 32203220.
- Xu Z, Pothula SP, Wilson JS, and Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 20: 11216-11229, 2014. PMID: 25170206.
- Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS, and Apte MV. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol 177: 2585-2596, 2010. PMID: 20934972.
- Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, Edderkaoui M, Pandol SJ, Park W, and Habtezion A. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology 151: 1206-1217, 2016. PMID: 27769811.
- Xue R, Yang J, Wu J, Meng Q, and Hao J. Coenzyme Q10 inhibits the activation of pancreatic stellate cells through PI3K/AKT/mTOR signaling pathway. Oncotarget 8: 92300-92311, 2017. PMID: 29190916.
- Yamamoto K, Tateishi K, Kudo Y, Hoshikawa M, Tanaka M, Nakatsuka T, Fujiwara H, Miyabayashi K, Takahashi R, Tanaka Y, Ijichi H, Nakai Y, Isayama H, Morishita Y, Aoki T, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M, and Koike K. Stromal remodeling by the BET bromodomain inhibitor JQ1 suppresses the progression of human pancreatic cancer. Oncotarget 7: 61469-61484, 2016. PMID: 27528027.
- Yan B, Cheng L, Jiang Z, Chen K, Zhou C, Sun L, Cao J, Qian W, Li J, Shan T, Lei J, Ma Q, and Ma J. Resveratrol Inhibits ROS-Promoted Activation and Glycolysis of Pancreatic Stellate Cells via Suppression of miR-21. Oxid Med Cell Longev 2018: 1346958, 2018. PMID: 29854071.
- Yan Z, Ohuchida K, Fei S, Zheng B, Guan W, Feng H, Kibe S, Ando Y, Koikawa K, Abe T, Iwamoto C, Shindo K, Moriyama T, Nakata K, Miyasaka Y, Ohtsuka T, Mizumoto K, Hashizume M, and Nakamura M. Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis. J Exp Clin Cancer Res 38: 221, 2019. PMID: 31133044.
- Yoshida N, Masamune A, Hamada S, Kikuta K, Takikawa T, Motoi F, Unno M, and Shimosegawa T. Kindlin-2 in pancreatic stellate cells promotes the progression of pancreatic cancer. Cancer Lett 390: 103-114, 2017. PMID: 28093281.
- Yoshida S, Yokota T, Ujiki M, Ding XZ, Pelham C, Adrian TE, Talamonti MS, Bell RH, Jr., and Denham W. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun 323: 1241-1245, 2004. PMID: 15451430.
- Yuzawa S, Kano MR, Einama T, and Nishihara H. PDGFRbeta expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol 29: 2824-2830, 2012. PMID: 22403002.
- Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S, Torres-Hernandez A, Tippens D, Pushalkar S, Eisenthal A, Saxena D, Ahn J, Hajdu C, Engle DD, Tuveson D, and Miller G. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med 212: 2077-2094, 2015. PMID: 26481685.
- Zha M, Xu W, Jones PM, and Sun Z. Isolation and characterization of human islet stellate cells. Exp Cell Res 341: 61-66, 2016. PMID: 26546984.
- Zhang H, Wu H, Guan J, Wang L, Ren X, Shi X, Liang Z, and Liu T. Paracrine SDF-1alpha signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget 6: 3085-3097, 2015. PMID: 25609203.
- Zhang X, Hu J, Zhuo Y, Cui L, Li C, Cui N, and Zhang S. Amygdalin improves microcirculatory disturbance and attenuates pancreatic fibrosis by regulating the expression of endothelin-1 and calcitonin gene-related peptide in rats. J Chin Med Assoc 81: 437-443, 2018. PMID: 29129515.
- Zhang Y, Yue D, Cheng L, Huang A, Tong N, and Cheng P. Vitamin A-coupled liposomes carrying TLR4-silencing shRNA induce apoptosis of pancreatic stellate cells and resolution of pancreatic fibrosis. J Mol Med (Berl) 96: 445-458, 2018. PMID: 29589070.
- Zhao L and Burt AD. The diffuse stellate cell system. J Mol Histol 38: 53-64, 2007. PMID: 17294244.
- Zimmermann A, Gloor B, Kappeler A, Uhl W, Friess H, and Buchler MW. Pancreatic stellate cells contribute to regeneration early after acute necrotising pancreatitis in humans. Gut 51: 574-578, 2002. PMID: 12235083.