Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2013.7
| Attachment | Size |
|---|---|
| 716.44 KB |
Gene symbols: ATP2B1, ATP2B2, ATP2B3, ATP2B3
1. General Information
The plasma membrane Ca2+-ATPase (PMCA) is an ATP-driven Ca2+ pump ubiquitously expressed in the plasma membrane of all eukaryotic cells. The PMCA is the major Ca2+ efflux pathway in non-excitable cells, such as pancreatic acinar cells, where the Na+/Ca2+-exchanger (NCX) is either not expressed or has minimal functional role. Even in cells where NCX is expressed abundantly, PMCA is critical for maintaining cytosolic Ca2+ concentration ([Ca2+]i) below 300 nM (~100 nM), due to its high affinity for Ca2+ (Kd, ~0.2 µM) (22, 23, 109).
Compared to other components of the Ca2+ signalling machinery the PMCA has received very little attention. This is because for many years the PMCA was thought to only have a minor house-keeping role in maintaining low resting [Ca2+]i with very little role in regulating dynamic Ca2+ signalling. However, in recent years the importance of PMCA in the spatiotemporal shaping of cytosolic Ca2+ signalling has steadily increased. For example, PMCA exhibits memory of past [Ca2+]i increases (25), suggesting an important role in regulating the frequency of Ca2+ oscillations. Moreover, the different PMCA isoforms, and numerous splice variants of PMCA, can be differentially expressed in specific regions of cells and can also be differentially regulated by a sophisticated repertoire of additional signalling pathways (22, 23).
Nevertheless, despite this emerging role of the PMCA in dynamic Ca2+ signalling, the importance of the house-keeping role of the PMCA must not be underestimated, especially when one considers how important maintaining low resting [Ca2+]i is for cell survival during pancreatic diseases such as pancreatitis, pancreatic cancer and diabetes. PMCA can be regarded as the last gatekeeper for the maintenance of low resting [Ca2+]i; even if all other Ca2+ clearance pathways are inhibited, if the PMCA is maintained or protected, cytosolic Ca2+ will nearly always recover giving the cell time to activate appropriate stress response pathways or even initiate apoptosis, which is generally protective. However, even if other Ca2+ clearance pathways are active, if PMCA is inhibited then the Ca2+ essentially has nowhere else to go, inevitably leading to an irreversible Ca2+ overload and the consequent necrotic cell death.
Structural features of the PMCA give rise to the functional diversity
PMCA is encoded by four separate genes (PMCA1-4) and numerous splice variants that give rise to specific tissue distribution, cellular localisation and functional diversity (76, 108). PMCA1 and PMCA4 are ubiquitously expressed whereas PMCA2 and PMCA3 have a more tissue specific expression and tend to be more abundant in excitable cells (49). Structurally, PMCA consists of 10 transmembrane domains, 2 cytosolic loops with both N- and C-terminal cytosolic tails (see Figure 1) (76, 108). The N-terminal tail exhibits the greatest diversity between the different isoforms and has been used to generate isoform-specific antibodies (107). The only functional feature of the N-terminal domain is the recently discovered 14-3-3-binding domain which has been shown to be inhibitory (71, 97).
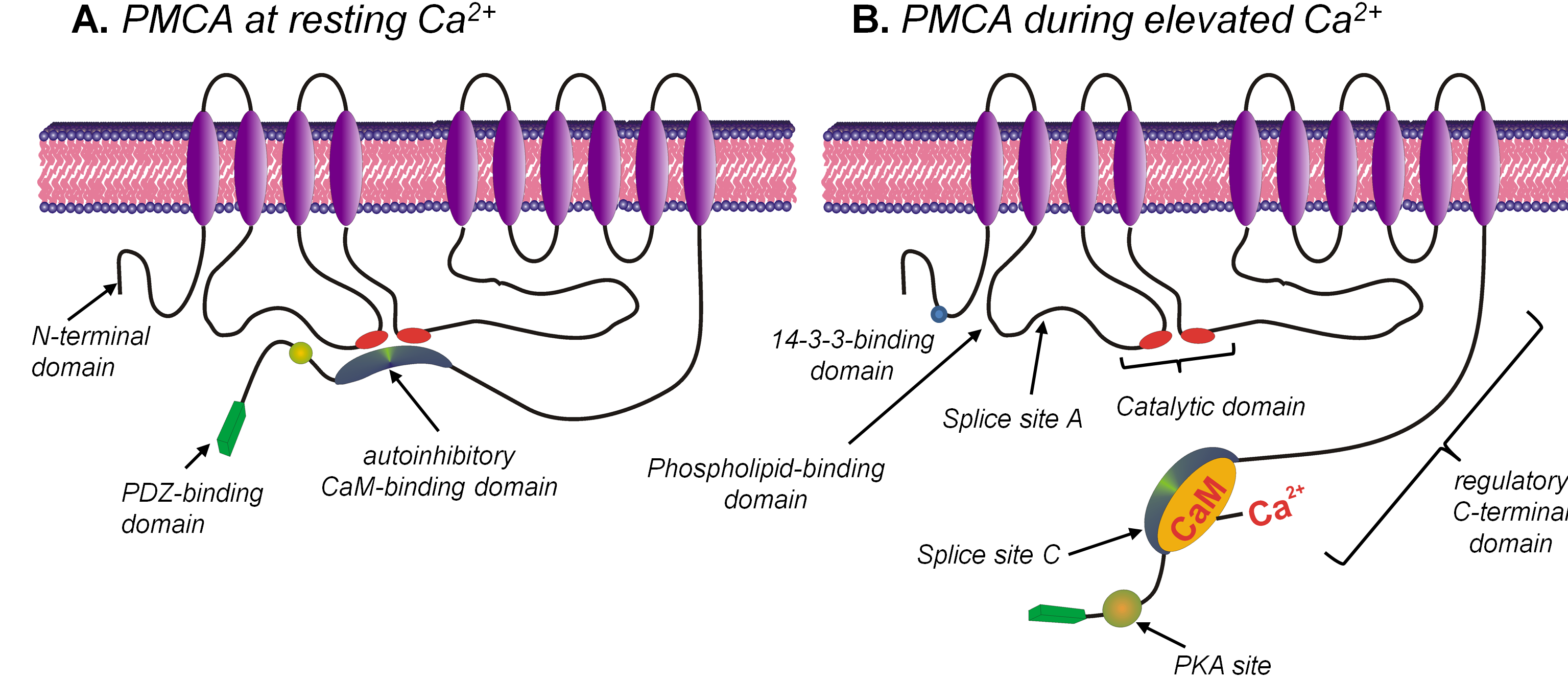
Figure 1. Cartoon depicting the main structural features of the PMCA at resting [Ca2+]i (A) and during elevated [Ca2+]i (B). The PMCA consists of an N-terminal domain, 10 transmembrane domains, 4 cytosolic loops and a regulatory C-terminal domain. When [Ca2+]i is elevated the Ca/CaM binds to the autoinhibitory CaM-binding domain causing a conformational change which exposes the catalytic site which consists of the ATP-binding sites and the aspartate residue that is phosphorylated during the reaction cycle. This increases the Ca2+-transporting activity of the PMCA.
The first cytosolic loop of the PMCA, which spans between the second and third transmembrane domains, contains numerous key functional domains. These include a stimulatory acidic phospholipid-binding domain (10, 121), part of the binding site for the autoinhibitory calmodulin (CaM)-binding domain (41) (see Figure 1B) and splice site A important for the apical membrane targeting in epithelial cells (28, 48, 57). The second cytosolic loop between the fourth and fifth transmembrane domains contains the major catalytic site. This includes the aspartate residue that becomes phosphorylated during the reaction cycle, the ATP binding domain and the second part of the binding site for the autoinhibitory CaM-binding domain within the C-terminal tail (40). Finally the C-terminal tail contains important regulatory domains of the PMCA, which includes the autoinhibitory CaM-binding domain (59), which at rest interacts with the catalytic domain thereby inhibiting the PMCA (Figure 1B). Binding of Ca2+/CaM to this autoinhibitory domain induces a conformational change which reduces its affinity for the catalytic site thereby increasing the Ca2+ transporting activity of the PMCA (22).
The C-terminal tail also contains consensus sites for phosphorylation by PKC (76, 91) and PKA (108), which when phosphorylated increases PMCA activity (44, 78, 105, 122) by increasing CaM binding (50). It has been hypothesised that due to the close proximity of the PKA site to the autoinhibitory CaM-binding domain (60), binding of CaM allows the otherwise cryptic PKA site to be accessible for PKA-mediated phosphorylation (58, 113). Indeed our own work in salivary acinar cells has shown that the apical PMCA activity is differentially potentiated and phosphorylated in a Ca2+-dependent manner (2, 13, 15). The C-terminus also contains additional high affinity allosteric Ca2+ binding sites (58) and acidic phospholipid binding site (79, 80). Binding of acidic phospholipids such as phosphatidylinositol (PI) and phosphatidylserine (PS) increases the Ca2+ and ATP affinity of PMCA. Phosphoinositide 4,5-bisphosphate (PIP2) is also a major activator of PMCA and is thought to account for ~50% of the activity of PMCA at rest (22, 23). Therefore, PIP2 depletion during G-protein-coupled receptor (GPCR) activation is likely to facilitate the increase in [Ca2+]i by inhibiting PMCA (22, 23) as well as generating IP3 and activating IP3-mediated Ca2+ release. The C-terminus also contains a critical splice site, which generates a truncated variant (a-variant) which has a reduced CaM affinity to the full length variant (b-variant) (Figure 1) (38). The functional significance of this is unclear as both splice variants are equally effective at restoring resting [Ca2+]i (9). The last few amino acid residues of the C-terminus of the PMCA contain PDZ-binding domains, which facilitate PMCA dimerisation (114) which further increases PMCA activity (67). In addition, the PDZ-binding domain also facilitates the recruitment of the actin cytoskeleton (118) and numerous scaffolding proteins and signalling complexes. These include; MAGUK (36), SAP (63), CASK (100), NHERF/EBP50 (35), PISP (47) and Ania3/Homer (104). Such targeting only occurs for the full-length b-variants, suggesting specialised signalling roles for different PMCA isoforms. Specifically, PMCA4b functionally interacts with nNOS (81, 101) and calcineurin (18) thereby regulating their downstream signalling pathways.
ATP dependency of PMCA
Intuitively, one might predict that ATP depletion would inhibit PMCA leading to Ca2+ overload and necrotic cell death (4, 26, 32). However, this is likely to be an over simplification because there are numerous factors that can influence the ATP sensitivity of the PMCA and also several factors that often accompany ATP depletion that can also separately influence PMCA activity.
Early studies revealed that PMCA has a catalytic ATP binding site (Km~3 µM) and lower affinity regulatory binding site (Km~145 µM) (96). However, more recent studies suggest a more complex ATP dependency (37). Most healthy cells have a resting ATP concentration in the mM range, which raises the important question of how much ATP has to drop before the PMCA is inhibited? Presumably this will depend on the extent of metabolic stress, whether mitochondria or glycolysis is inhibited, the overall metabolic activity of the cell and whether ATP is being rapidly consumed. Secondly, would inhibition of mitochondrial metabolism alone be enough to deplete ATP levels sufficiently to inhibit the PMCA activity especially if glycolytic ATP production remains active? The classic textbook view is that mitochondria contribute ~95 % of ATP (i.e. 32 molecules of ATP per glucose molecule), whereas glycolysis provides only 5 % (i.e. 2 molecules of ATP per glucose molecule). This is likely to be an over simplification as most cells exhibit metabolic plasticity and are able to adapt to their environment, for example during hypoxia. Cancer cells are an extreme example of this and often undergo a dramatic switch from mitochondrial metabolism to glycolytic metabolism due to mutations of key mitochondrial enzymes and up-regulation of glycolytic enzymes (89, 90). This is known as the Warburg effect, named after Otto Warburg, who first described this in the 1920s (115). Our ongoing recent work on pancreatic cancer cell lines show that glycolytic inhibitors induce cytotoxic Ca2+ overload, ATP depletion, inhibition of the PMCA and the consequent necrotic cell death, whereas mitochondrial inhibitors had no effect (unpublished data). This suggests that glycolytically-derived ATP, rather than mitochondrially-derived ATP is critical for maintaining PMCA function and pancreatic cancer cell survival.
Regulation of the PMCA by acidic phospholipids
An important caveat when considering the ATP-sensitivity of the PMCA is that acidic phosphoplipids, such as phosphatidylinositol (PI) and phosphatidylserine (PS) increase the ATP sensitivity of the PMCA and mimic regulation by CaM (79, 80). In particular, the affinity for ATP was as low as 5-10 mM (regulatory site) when PS (or PI) was absent from the lipid environment of the PMCA using cell-free assays (69, 98). This therefore suggests that depletion of PS (or PI) from the membrane may be sufficient to render the PMCA highly sensitive to ATP depletion. However, most of the evidence is based on in vitro cell-free assays of ATPase activity, whereby PS/PI was either absent or present in an artificial membrane, which makes it difficult to extrapolate to intact cells. It is therefore unclear what the critical concentration of PS is to maintain “normal” ATP-sensitivity of the PMCA or whether this relationship is influenced by dynamic changes in Ca2+, Mg2+, CaM or lipid environment. However, functional studies in intact endothelial cells have shown that the loss of phosphatidylserine from the inner leaflet of the plasma membrane, following cholesterol depletion with β-methyl-cyclodextran, inhibited PMCA activity (120). This has important implication for apoptosis, since PS is known to line the inner leaflet of the plasma membrane and a proportion is thought to flip to the extracellular side of the membrane during apoptosis (39). This provides the dying cell with an “eat me” signal detected by macrophages that then phagocytose the dying cell from the tissue (39). Furthermore, the enzyme responsible for this PS asymmetry within the plasma membrane (aminophospholipid translocase or flippase) (34), requires millimolar ATP (103, 106) and is inhibited by oxidative stress (16, 56). Collectively these studies suggest that cellular stress may have a profound effect on the ATP sensitivity of the PMCA and thus inhibit the PMCA even with only mild ATP depletion. However, more work is needed to fully understand the complex relationship between membrane phospholipids, ATP and PMCA activity.
Effect of mitochondrially-derived reactive oxygen species
Severe mitochondrial stress, whatever the mechanism, often leads to the generation of reactive oxygen species (ROS) (95). ROS are generated by incomplete reduction of oxygen during the process of oxidative metabolism. In fact, ~1–5% of electrons ‘escape’ the electron transport chain to generate superoxide (O2.-) (95). The principal source of superoxide is complex III (66) and complex I (19) which in turn can be converted to H2O2 by superoxide dismutase and released by mitochondria. There is also good evidence that oxidants (H2O2) can directly oxidise PMCA and also oxidise calmodulin, which is the main activator of PMCA (119). Hence, metabolically derived ROS may have a profound inhibitory effect on PMCA activity.
In addition, H2O2 has been reported to reduce the functional expression of PMCA at the plasma membrane of cultured hippocampal neurons within 1-2 hours (65). Such rapid changes in functional expression of PMCA at the plasma membrane could lead to reduced Ca2+ efflux during metabolic stress even in the presence of continued high ATP levels.
Calpain/caspase cleavage of the PMCA
The release of cytochrome C from the mitochondria and the subsequent activation of caspases and calpain (55) have both been reported to cleave and eventually lead to the inactivation of the PMCA (11, 51, 102). It is interesting to note that the time-frame over which cytochrome C release can occur (> 2 mins) (46) coincides with the time the PMCA can be observed to be inhibited, and well before ATP depletion was observed (3).In fact the initial cleavage by caspase and calpain actually activates the pump, but through the subsequent internalisation and degradation of the PMCA the protease effect is manifested as pump inhibition (27, 92). Specific caspase-3 cleavage of PMCA4b produces a 120 kD fragment that is constitutively activated, due to the removal of the autoinhibitory domain (85-87). It is also interesting to note that calpain can also be directly activated by H2O2 and Ca2+ (116).
2. PMCA in the pancreas
Compared to the study of other Ca2+ transport pathways, such as IP3 receptors, store-operated Ca2+ entry (SOCE) and sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), there has been very little work focussing on PMCA function in pancreatic exocrine cells. Early in vitro studies of ATPase activity and Ca2+ flux in isolated pancreatic acinar cell membranes revealed that the PMCA has a high affinity for Ca2+ (Kd, ~2 µM) and is activated by CaM, PKA, PKC and phospholipids, consistent with studies in red blood cell membranes (1, 62, 72, 73). However, it is difficult to translate these findings to live intact cells due to the dynamic changes in cytosolic Ca2+, ATP, phospholipid composition of the membrane and other signalling molecules. Therefore, studies in intact pancreatic acinar cells have confirmed some of these early findings and importantly have revealed that PMCA was the major, if not only, Ca2+ efflux pathway in pancreatic acinar cells due to the lack of functional expression of the Na+/Ca2+-exchange (NCX) (77). This was later confirmed using the droplet technique; an elegant approach for visualising Ca2+ efflux from single cells or clusters of cells, using dextran-conjugated fluorescent dyes trapped within a very small extracellular volume beneath a droplet of oil (110, 111). Using this technique, Ca2+ efflux via the PMCA, appeared to be much greater at the apical membrane of pancreatic acinar cells (5-7). Moreover, immunofluorescence studies confirmed that the PMCA is expressed with a much higher density at the apical and lateral membranes of pancreatic acinar cells (68). This apically confined Ca2+ efflux occurred during each apically-confined, IP3R-mediated, cytosolic Ca2+ spike during agonist-evoked [Ca2+]i oscillations (110, 111). This led to the “Ca2+ tunnelling” hypothesis, proposed by Ole Petersen’s group (75). Since apically confined Ca2+ release leads to apical Ca2+ efflux, via the PMCA, the ER needs to be replenished to sustain these apically confined [Ca2+]i oscillations. This was shown to occur by Ca2+ entry across the basolateral membrane and the subsequent re-uptake of Ca2+ into the ER by basolateral SERCA, thus allowing Ca2+ to “tunnel” through to the ER thereby facilitating regenerative apical Ca2+ release (75). Furthermore, this apical Ca2+ efflux into the pancreatic duct lumen activates Ca2+-sensing receptors (CaSR) which line the apical membrane of pancreatic duct cells thereby facilitating bicarbonate and thus fluid secretion (14). This has been suggested to be an important protective mechanism controlling the lithogenicity of pancreatic fluid and preventing pancreatic stone formation (14).
In the related salivary acinar cells we have also shown that the apical PMCA activity could be differentially potentiated by a Ca2+-dependent, PKA-mediated phosphorylation also important for facilitating maximum fluid secretion (2). Taken together these studies have elevated the importance of the PMCA from a minor house-keeping role to critical roles in the spatiotemporal shaping of [Ca2+]i signalling and the control of fluid secretion and lithogenecity in the pancreas and salivary glands.
In pancreatic islets all 4 isoforms, and numerous splice variants, of the PMCA have been shown to be expressed, using immunocytochemistry, western blotting and RT-PCR (45, 61, 99, 112). Specifically, PMCA 1 and 4 are ubiquitously expressed in all cells of the islets, whereas PMCA3 and the splice variants PMCA1b, PMCA2b and PMCA4b are reported to be exclusively expressed in β-cells (45, 61, 112). Interestingly it has been shown functionally that elevated glucose has an inhibitory effect on PMCA activity in rat pancreatic islets/β-cells (99, 117). Although the mechanism for this inhibition remains obscure it has been suggested that there is a switch from PMCA function as the major Ca2+ efflux mechanism (at low [Ca2+]i) to NCX function (at high [Ca2+]i). It is also important to note that many of these studies use cell free assays of ATPase activity as a measure of PMCA, which makes it difficult to extrapolate PMCA function to intact live cells.
PMCA and cell death
Although the physiological role of the PMCA has been debated for several years, the role of the PMCA during cellular stress and under pathological conditions is undeniable. The nature of cell death (i.e. necrosis vs apoptosis) will largely depend on the extent of metabolic stress and cytosolic Ca2+ overload. However, ATP depletion during extensive metabolic stress has been suggested to be the switch from apoptosis to necrosis (31, 64, 70), regardless of whether this is accompanied by Ca2+ overload. This is largely because ATP is required for many of the apoptotic processes, but not for necrosis (70). During pancreatitis, apoptosis is generally regarded as protective as this involves the safe dismantling of the cell constituents (8, 52). Necrosis, on the other hand, is the uncontrolled cell death due to cell lysis and the release of activated proteases (zymogens) which trigger the spiral of self-perpetuating tissue damage characteristic of acute pancreatitis (8, 52). This includes local inflammation and the recruitment of activated neutrophils to the site of cellular injury. This often leads to systemic inflammation especially if the activated zymogens leak into the blood stream causing distal organ damage and thus multiple organ failure (84). Cytosolic Ca2+ overload, metabolic stress and necrosis are linked in various ways, but perhaps most critically via the PMCA.
In addition, in the context of cell death and cytoprotection, the impairment of PMCA function and subsequent dysregulation of cytosolic Ca2+ homeostasis can, in some cases, be cyto-protective (83). During oxidative stress or tumour necrosis factor (TNF)-induced cell death, the accumulation and damage of lysosomes has been suggested to be important (17, 82). In TNF-resistant cell lines, in which PMCA4 is mutated, the resulting enhanced Ca2+ signalling has been shown to promote the exocytotic loss of lysosomes resulting in protection against TNF-induced cell death (83). This therefore suggests, somewhat counter-intuitively, that PMCA4 promotes TNF-induced cell death.
Interestingly, the anti-apoptotic factor Bcl-2/xL has been recently shown to inhibit PMCA activity in pancreatic acinar cells (42). Although the pathophysiological relevance of this phenomena remains to be determined this observation highlights the critical importance of the PMCA in controlling cell fate.
Effect of ATP depletion on PMCA activity during metabolic stress
This will depend on the “extent” of metabolic stress, whether mitochondria or glycolysis is inhibited and how quickly ATP is consumed. We have previously reported that oxidative stress, induced by H2O2, impaired hormone evoked Ca2+ oscillations, induced an irreversible Ca2+ overload and marked inhibition of PMCA in pancreatic acinar cells (3, 12). This H2O2-induced PMCA inhibition could occur without mitochondrial Ca2+ handling, coincided with mitochondrial depolarization and was sensitive to inhibitors of the mitochondrial permeability transition pore (mPTP) (3). These data were consistent with studies showing that severe ATP depletion can cause inhibition of the PMCA (4, 26, 32). PMCA activity was shown to decrease ~5 fold following the combined treatment with oligomycin and iodoacetate in pancreatic acinar cells (4). Moreover, the pancreatitis-inducing agents, palmitoleic acid ethylester (POAEE), palmitoleic acid (POA) and bile acids have all been shown to induce Ca2+ overload, mitochondrial depolarisation and ATP depletion (32). This POAEE-induced Ca2+ overload, which was due to ATP-depletion-induced inhibition of the PMCA and SERCA, was largely abrogated by replenishment of ATP via a patch pipette (32). However, our recent studies have shown that the H2O2-induced Ca2+ overload, ATP depletion and inhibition of the PMCA were abrogated by pre-treatment with insulin (74), which has recently been extended to POA-induced cytotoxicity (unpublished data). This insulin protection was independent of oxidative stress and mitochondrial depolarisation but involved activation of PI3K/Akt and a switch from mitochondrial metabolism to glycolytic metabolism which was sufficient to preserve the ATP supply to the PMCA, thereby preventing cytotoxic Ca2+ overload (74).
Incidentally the PMCA has been shown to have its own localised glycolytic ATP supply, which under certain conditions may render it largely insensitive to inhibition of mitochondrial metabolism (53, 54, 88). Specifically, studies in isolated inside-out plasma membrane vesicles from pig stomach smooth muscle enriched with PMCA showed that an endogenous membrane-bound glycolytic system provided ATP to fuel the PMCA-dependent Ca2+ uptake (53, 54). As long as glycolytic substrates were present the Ca2+ uptake (PMCA activity) persisted in the absence of an exogenously applied ATP regenerating system (53, 54). Furthermore, studies in red blood cells have shown that several glycolytic enzymes associate with the plasma membrane, either via band 3 protein (anion-exchanger) (20, 21, 29, 94) or via phospholipids (33). Moreover, the PMCA has been shown to reside within caveolae, where these phospholipids are enriched and regulate the activity of the PMCA (120). Finally, it has been suggested that a localised pool of ATP, associated with the cytoskeleton, provides a privileged ATP supply to the PMCA (30). Collectively these data have important implications for the ATP-regulation of the PMCA.
Summary
The PMCA is critical for maintaining low resting cytosolic Ca2+ and for preventing cytotoxic Ca2+ overload and cell death, particularly in cells in which the NCX is either not expressed or has minimal functional activity, such as pancreatic acinar cells. In addition, there is increasing evidence that the PMCA may physically and functionally couple to a variety of signalling pathways and metabolic enzymes, elevating its importance as a general house-keeping role to the control of dynamic cell signalling in both the exocrine and endocrine pancreas. Since the PMCA is an ATP-driven pump, severe metabolic stress and ATP depletion during pathological situations should lead to inhibition of the PMCA and thus Ca2+ overload and cell death. Although this relationship is likely an over-simplification the importance of the PMCA in diseases such as pancreatitis, pancreatic cancer and diabetes warrant further investigation.
3. Tools available to study the PMCA
a. cDNA clones
A variety of PMCA containing plasmids can be obtained from Emanuel Strehler (Mayo Clinic, Rochester, MN). These include DNA constructs encoding EGFP fused to PMCA2b or PMCA4b (36) and DNA constructs encoding the truncated PMCA that lacks the PDZ-binding domain (EGFP-PMCA2b6 & EGFP-PMCA4b6) (28).
b. Antibodies
Non isoform-specific PMCA ATPase Antibody (clone 5F10) (Mouse monoclonal; MA3-914; Thermo Scientific, Pierce Antibodies)
PMCA1-specific ATPase Antibody (clone 5F10) (rabbit polyclonal; PA1-914; Thermo Scientific, Pierce Antibodies)
PMCA2-specific ATPase Antibody (rabbit polyclonal; PA1-915; Thermo Scientific, Pierce Antibodies)
PMCA3-specific ATPase Antibody (rabbit polyclonal; PA1-916; Thermo Scientific, Pierce Antibodies)
PMCA4-specific ATPase Antibody (clone JA9) (Mouse monoclonal; MA1-914; Thermo Scientific, Pierce Antibodies). JA9 reacts specifically with a region containing residues 51–75 of hPMCA4 (a or b), but not with the same region of PMCA1, 2 or 3 (24).
Santa Cruz Biotech has produced a range of PMCA4b-specific mouse monoclonal, including clone JA3, 2T2 and 3F18. JA3 reacted with residues 1156–1180, a region unique to hPMCA4b (24).
Other splice variant specific polyclonal antibodies that recognise the a-splice variants of PMCA1, 2, 3 and 4 directed against the C-terminal domains of each PMCA isoform termed CR1a, CR2a, CR3a and CR4a and thus recognise PMCA1a, 2a, 3a and 4a respectively have been described (43).
c. Mouse Models
A range of PMCA knockout mice have been created by numerous groups but most noticeably by Gary Schull (University of Cincinnati College of Medicine, Cincinnati, Ohio) (93) and Lugwig Neyses (University of Manchester, UK). The CByJ.A-Atp2b2dfw-2J/J mouse strain which represents the PMCA2 knockout can be obtained from Jackson Labs (http://jaxmice.jax.org/strain/002894.html)
d. In situ Ca2+ clearance Assay
We have developed an in situ [Ca2+]I clearance assay in which all other Ca2+ transport is inhibited in live intact cells. While in vitro cell-free assays of ATPase activity and 45Ca2+ flux measurements have provided valuable information in characterising the PMCA function, these techniques are severely limited. This is because PMCA activity in a live cell can be influenced by dynamic changes in Ca2+, ATP, numerous cytosolic factors and the phospholipid environment within the plasma membrane. Further details of our in situ [Ca2+]I clearance assay can be found in reference 2 and 68 and figure 2 below.

Figure 2. In situ [Ca2+]i clearance assay of PMCA activity in live intact pancreatic acinar cells. This involves treating cells with 30 µM cyclopiazonic acid (CPA) in zero external Ca2+ (1 mM EGTA), which inhibits SERCA, thereby depleting the ER of Ca2+. This lead to a small increase in [Ca2+]i which recovered to baseline presumably due to the PMCA. The addition of 20 mM Ca2+ leads to a large increase in [Ca2+]i due to store-operated Ca2+ entry which then reaches a short-lived steady state. Subsequent removal of external Ca2+ (1 mM EGTA) leads to clearance of [Ca2+]i due predominantly to the PMCA. Mitochondrial Ca2+ uptake may contribute to this net [Ca2+]i clearance but this can be abolished using the mitochondrial Ca2+ uptake inhibitor, Ru360 (see Ref 2). This influx-clearance phase can be repeated in the presence of a test reagent/treatment using a paired experimental design and the rate normalised to the initial clearance of the same cell. This normalised rate can then be compared to time-matched control experiments (see Ref 3 and 74).
e. Characterisation of PMCA activity in pancreatic acinar cells
We have shown that following treatment with the SERCA inhibitor, cyclopiazonic acid (CPA), [Ca2+]i clearance was inhibited using the PMCA-specific inhibitor, carboxyeosin diacetate (Figure 3b). Under conditions of the in situ [Ca2+]i clearance assay the NCX has no role, as [Ca2+]i clearance was unaffected by replacing all external Na2+ with N-methyl-D-glucamine (NMDG) (Figure 3D). Further details can be found in reference 2 and 68 and figure 3 below.
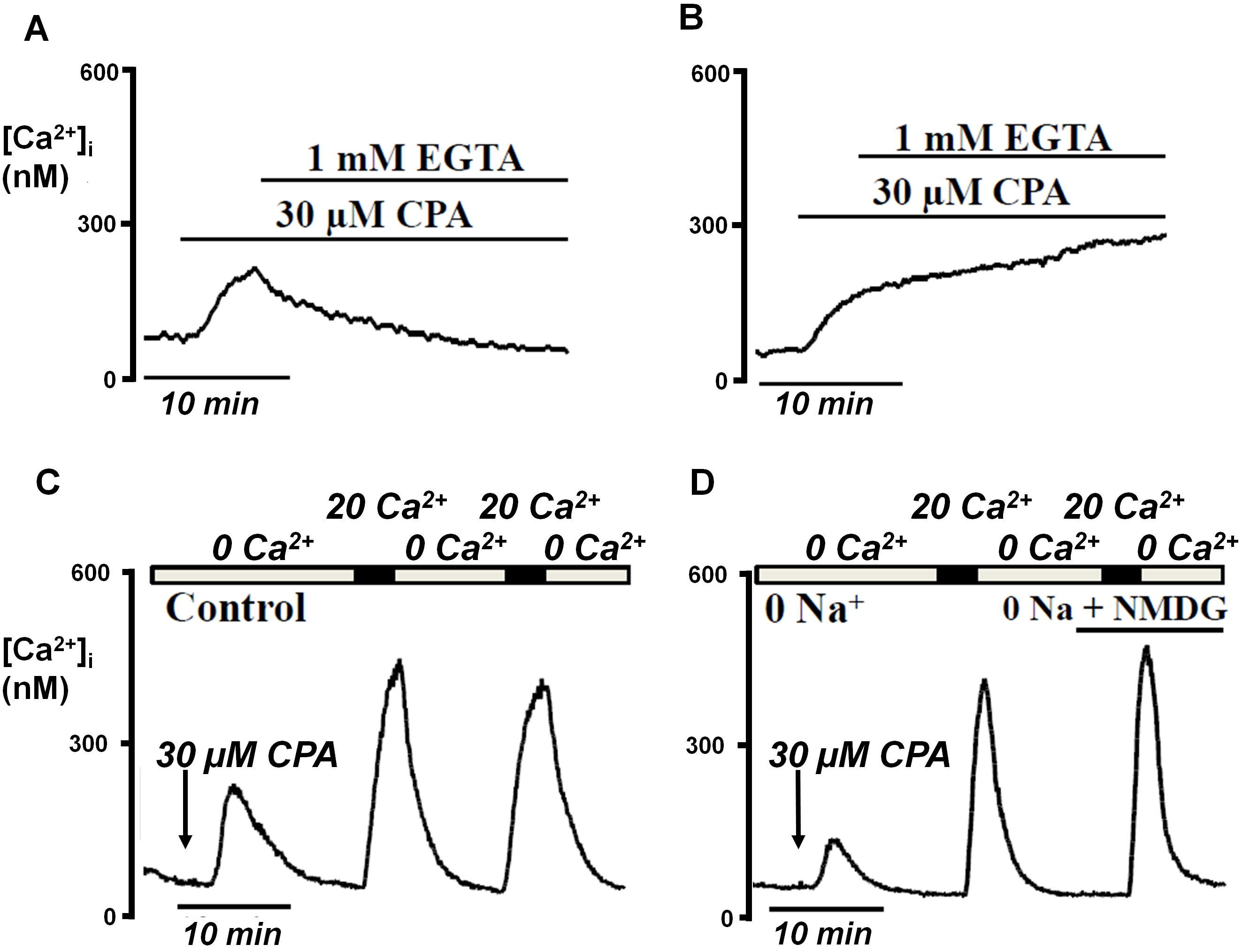
Figure 3.Cartoon depicting the main structural features of the PMCA at resting [Ca2+]i (A) and during elevated [Ca2+]i (B). Characterisation of PMCA activity in pancreatic acinar cells. Cell were pre-incubated without (A) or with (B) carboxyeosin diacetate to inhibit the PMCA. Treatment with CPA caused a leak of Ca2+ from the ER and subsequent activation of SOCE. Subsequent removal of external Ca2+ (1 mM EGTA) caused [Ca2+]i to be rapidly cleared from the cytosol of control cells (A) but not from carboxyeosin-treated cells (B). Using the in situ [Ca2+]i clearanceassay, replacing all external Na+ with N-methyl-D-glucamine (NMDG) had no effect on the rate of clearance, suggesting that the NCX has no role.
4. References
- Ansah TA, Molla A, and Katz S. Ca2+-ATPase activity in pancreatic acinar plasma membranes. Regulation by calmodulin and acidic phospholipids. J Biol Chem 259: 13442-13450, 1984. PMID: 6149223
- Baggaley E, McLarnon S, Demeter I, Varga G, and Bruce JI. Differential regulation of the apical plasma membrane Ca(2+) -ATPase by protein kinase A in parotid acinar cells. J Biol Chem 282: 37678-37693, 2007. PMID: 17938178
- Baggaley EM, Elliott AC, and Bruce JI. Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: role of the mitochondria. Am J Physiol Cell Physiol 295: C1247-1260, 2008. PMID: 18787078
- Barrow SL, Voronina SG, da Silva Xavier G, Chvanov MA, Longbottom RE, Gerasimenko OV, Petersen OH, Rutter GA, and Tepikin AV. ATP depletion inhibits Ca(2+) release, influx and extrusion in pancreatic acinar cells but not pathological Ca(2+) responses induced by bile. Pflugers Arch 455: 1025-1039, 2008. PMID: 17952455
- Belan P, Gerasimenko O, Petersen OH, and Tepikin AV. Distribution of Ca2+ extrusion sites on the mouse pancreatic acinar cell surface. Cell Calcium 22: 5-10, 1997. PMID: 9232347
- Belan PV, Gerasimenko OV, Berry D, Saftenku E, Petersen OH, and Tepikin AV. A new technique for assessing the microscopic distribution of cellular calcium exit sites. Pflugers Arch 433: 200-208, 1996. PMID: 9019724
- Belan PV, Gerasimenko OV, Tepikin AV, and Petersen OH. Localization of Ca2+ extrusion sites in pancreatic acinar cells. J Biol Chem 271: 7615-7619, 1996. PMID: 8631796
- Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189-196, 2004. PMID: 14715516
- Brini M, Coletto L, Pierobon N, Kraev N, Guerini D, and Carafoli E. A comparative functional analysis of plasma membrane Ca2+ pump isoforms in intact cells. J Biol Chem 278: 24500-24508, 2003. PMID: 12716903
- Brodin P, Falchetto R, Vorherr T, and Carafoli E. Identification of two domains which mediate the binding of activating phospholipids to the plasma-membrane Ca2+ pump. Eur J Biochem 204: 939-946, 1992. PMID: 1311684
- Brown CS and Dean WL. Regulation of plasma membrane Ca2+-ATPase in human platelets by calpain. Platelets 18: 207-211, 2007. PMID: 17497432
- Bruce JI and Elliott AC. Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase. Am J Physiol Cell Physiol 293: C938-950, 2007. PMID: 18787078
- Bruce JI, Straub SV, and Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 34: 431-444, 2003. PMID: 14572802
- Bruce JI, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, and Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem 274: 20561-20568, 1999. PMID: 10400686
- Bruce JI, Yule DI, and Shuttleworth TJ. Ca2+-dependent protein kinase--a modulation of the plasma membrane Ca2+-ATPase in parotid acinar cells. J Biol Chem 277: 48172-48181, 2002. PMID: 12368283
- Brunauer LS, Moxness MS, and Huestis WH. Hydrogen peroxide oxidation induces the transfer of phospholipids from the membrane into the cytosol of human erythrocytes. Biochemistry 33: 4527-4532, 1994. PMID: 8161507
- Brunk UT and Svensson I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep 4: 3-11, 1999. PMID: 10714269
- Buch MH, Pickard A, Rodriguez A, Gillies S, Maass AH, Emerson M, Cartwright EJ, Williams JC, Oceandy D, Redondo JM, Neyses L, and Armesilla AL. The sarcolemmal calcium pump inhibits the calcineurin/nuclear factor of activated T-cell pathway via interaction with the calcineurin A catalytic subunit. J Biol Chem 280: 29479-29487, 2005. PMID: 15955804
- Cadenas E, Boveris A, Ragan CI, and Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Archives of biochemistry and biophysics 180: 248-257, 1977. PMID: 195520
- Campanella ME, Chu H, and Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci U S A 102: 2402-2407, 2005. PMID: 15701694
- Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, and Low PS. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood 112: 3900-3906, 2008. PMID: 18698006
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. Faseb J 8: 993-1002, 1994. PMID: 7926378
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev 71: 129-153, 1991. PMID: 1986387
- Caride AJ, Filoteo AG, Enyedi A, Verma AK, and Penniston JT. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem J 316 ( Pt 1): 353-359, 1996. PMID: 8645230
- Caride AJ, Penheiter AR, Filoteo AG, Bajzer Z, Enyedi A, and Penniston JT. The plasma membrane calcium pump displays memory of past calcium spikes. Differences between isoforms 2b and 4b. J Biol Chem 276: 39797-39804, 2001. PMID: 11514555
- Castro J, Ruminot I, Porras OH, Flores CM, Hermosilla T, Verdugo E, Venegas F, Hartel S, Michea L, and Barros LF. ATP steal between cation pumps: a mechanism linking Na+ influx to the onset of necrotic Ca2+ overload. Cell Death Differ 13: 1675-1685, 2006. PMID: 16410794
- Chami M, Ferrari D, Nicotera P, Paterlini-Brechot P, and Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem 278: 31745-31755, 2003. PMID: 12799372
- Chicka MC and Strehler EE. Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 278: 18464-18470, 2003. PMID: 12624087
- Chu H and Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J 400: 143-151, 2006. PMID: 16836485
- Chu H, Puchulu-Campanella E, Galan JA, Tao WA, Low PS, and Hoffman JF. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc Natl Acad Sci U S A 109: 12794-12799, 2012. PMID: 22745158
- Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, and Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ 14: 1285-1294, 2007. PMID: 17431416
- Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, and Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology 130: 781-793, 2006. PMID: 16530519
- Dabrowska A, Pietkiewicz J, Dabrowska K, Czapinska E, and Danielewicz R. Interaction of M1 and M2 isozymes pyruvate kinase from human tissues with phospholipids. Biochim Biophys Acta 1383: 123-129, 1998. PMID: 9546053
- Daleke DL. Phospholipid flippases. J Biol Chem 282: 821-825, 2007. PMID: 17130120
- DeMarco SJ, Chicka MC, and Strehler EE. Plasma membrane Ca2+ ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J Biol Chem 277: 10506-10511, 2002. PMID: 11786550
- DeMarco SJ and Strehler EE. Plasma membrane Ca2+-atpase isoforms 2b and 4b interact promiscuously and selectively with members of the membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1) domain-containing proteins. J Biol Chem 276: 21594-21600, 2001. PMID: 11274188
- Echarte MM, Rossi RC, and Rossi JP. Phosphorylation of the plasma membrane calcium pump at high ATP concentration. On the mechanism of ATP hydrolysis. Biochemistry 46: 1034-1041, 2007. PMID: 17240987
- Enyedi A, Verma AK, Heim R, Adamo HP, Filoteo AG, Strehler EE, and Penniston JT. The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem 269: 41-43, 1994. PMID: 8276828
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, and Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148: 2207-2216, 1992. PMID: 1545126
- Falchetto R, Vorherr T, Brunner J, and Carafoli E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J Biol Chem 266: 2930-2936, 1991. PMID: 1847139
- Falchetto R, Vorherr T, and Carafoli E. The calmodulin-binding site of the plasma membrane Ca2+ pump interacts with the transduction domain of the enzyme. Protein Sci 1: 1613-1621, 1992. PMID: 1339025
- Ferdek PE, Gerasimenko JV, Peng S, Tepikin AV, Petersen OH, and Gerasimenko OV. A novel role for Bcl-2 in regulation of cellular calcium extrusion. Curr Biol 22: 1241-1246, 2012. PMID: 22704985
- Filoteo AG, Elwess NL, Enyedi A, Caride A, Aung HH, and Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem 272: 23741-23747, 1997. PMID: 9295318
- Furukawa K, Tawada Y, and Shigekawa M. Regulation of the plasma membrane Ca2+ pump by cyclic nucleotides in cultured vascular smooth muscle cells. J Biol Chem 263: 8058-8065, 1988. PMID: 2836409
- Garcia ME, Del Zotto H, Caride AJ, Filoteo AG, Penniston JT, Rossi JP, and Gagliardino JJ. Expression and cellular distribution pattern of plasma membrane calcium pump isoforms in rat pancreatic islets. J Membr Biol 185: 17-23, 2002. PMID: 11891561
- Gerasimenko JV, Gerasimenko OV, Palejwala A, Tepikin AV, Petersen OH, and Watson AJ. Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci 115: 485-497, 2002. PMID: 11861756
- Goellner GM, DeMarco SJ, and Strehler EE. Characterization of PISP, a novel single-PDZ protein that binds to all plasma membrane Ca2+-ATPase b-splice variants. Ann N Y Acad Sci 986: 461-471, 2003. PMID: 12763866
- Grati M, Aggarwal N, Strehler EE, and Wenthold RJ. Molecular determinants for differential membrane trafficking of PMCA1 and PMCA2 in mammalian hair cells. J Cell Sci 119: 2995-3007, 2006. PMID: 16803870
- Greeb J and Shull GE. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem 264: 18569-18576, 1989. PMID: 2530223
- Gromadzinska E, Lachowicz L, Walkowiak B, and Zylinska L. Calmodulin effect on purified rat cortical plasma membrane Ca(2+)-ATPase in different phosphorylation states. Biochim Biophys Acta 1549: 19-31, 2001. PMID: 11566365
- Guerini D, Pan B, and Carafoli E. Expression, purification, and characterization of isoform 1 of the plasma membrane Ca2+ pump: focus on calpain sensitivity. J Biol Chem 278: 38141-38148, 2003. PMID: 12851406
- Gukovskaya AS, Mareninova OA, Odinokova IV, Sung KF, Lugea A, Fischer L, Wang YL, Gukovsky I, and Pandol SJ. Cell death in pancreatitis: effects of alcohol. Journal of gastroenterology and hepatology 21 Suppl 3: S10-13, 2006.
- Hardin CD, Raeymaekers L, and Paul RJ. Comparison of endogenous and exogenous sources of ATP in fueling Ca2+ uptake in smooth muscle plasma membrane vesicles. J Gen Physiol 99: 21-40, 1992. PMID: 1311020
- Hardin CD, Zhang C, Kranias EG, Steenaart NA, Raeymaekers L, and Paul RJ. Regulation of glycolytically fueled Ca2+ uptake in smooth muscle plasmalemmal vesicles by phosphorylation. Am J Physiol 265: H1326-1333, 1993. PMID: 8238421
- Harwood SM, Yaqoob MM, and Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem 42: 415-431, 2005. PMID: 16259792
- Herrmann A and Devaux PF. Alteration of the aminophospholipid translocase activity during in vivo and artificial aging of human erythrocytes. Biochim Biophys Acta 1027: 41-46, 1990. PMID: 2168752
- Hill JK, Williams DE, LeMasurier M, Dumont RA, Strehler EE, and Gillespie PG. Splice-site A choice targets plasma-membrane Ca2+-ATPase isoform 2 to hair bundles. J Neurosci 26: 6172-6180, 2006. PMID: 16763025
- Hofmann F, James P, Vorherr T, and Carafoli E. The C-terminal domain of the plasma membrane Ca2+ pump contains three high affinity Ca2+ binding sites. J Biol Chem 268: 10252-10259, 1993. PMID: 8387515
- James P, Maeda M, Fischer R, Verma AK, Krebs J, Penniston JT, and Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem 263: 2905-2910, 1988. PMID: 2963820
- James PH, Pruschy M, Vorherr TE, Penniston JT, and Carafoli E. Primary structure of the cAMP-dependent phosphorylation site of the plasma membrane calcium pump. Biochemistry 28: 4253-4258, 1989. PMID: 2548572
- Kamagate A, Herchuelz A, Bollen A, and Van Eylen F. Expression of multiple plasma membrane Ca(2+)-ATPases in rat pancreatic islet cells. Cell Calcium 27: 231-246, 2000. PMID: 10858669
- Katz S and Ansah TA. Ca2+-transport and Mg2+- and Ca2+-ATPase activity in the exocrine pancreas. Journal of pediatric gastroenterology and nutrition 3 Suppl 1: S11-18, 1984. PMID: 6150078
- Kim E, DeMarco SJ, Marfatia SM, Chishti AH, Sheng M, and Strehler EE. Plasma membrane Ca2+ ATPase isoform 4b binds to membrane-associated guanylate kinase (MAGUK) proteins via their PDZ (PSD-95/Dlg/ZO-1) domains. J Biol Chem 273: 1591-1595, 1998. PMID: 9430700
- Kim JS, Qian T, and Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology 124: 494-503, 2003. PMID: 12557154
- Kip SN and Strehler EE. Rapid downregulation of NCX and PMCA in hippocampal neurons following H2O2 oxidative stress. Ann N Y Acad Sci 1099: 436-439, 2007. PMID: 17446483
- Korshunov SS, Skulachev VP, and Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15-18, 1997. PMID: 9369223
- Kosk-Kosicka D and Bzdega T. Activation of the erythrocyte Ca2+-ATPase by either self-association or interaction with calmodulin. J Biol Chem 263: 18184-18189, 1988. PMID: 2973461
- Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, and Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15771-15776, 1997. PMID: 9188473
- Lehotsky J, Raeymaekers L, Missiaen L, Wuytack F, De Smedt H, and Casteels R. Stimulation of the catalytic cycle of the Ca2+ pump of porcine plasma-membranes by negatively charged phospholipids. Biochim Biophys Acta 1105: 118-124, 1992. PMID: 1314667
- Leist M, Single B, Castoldi AF, Kuhnle S, and Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a swit ch in the decision between apoptosis and necrosis. J Exp Med 185: 1481-1486, 1997. PMID: 9126928
- Linde CI, Di Leva F, Domi T, Tosatto SC, Brini M, and Carafoli E. Inhibitory interaction of the 14-3-3 proteins with ubiquitous (PMCA1) and tissue-specific (PMCA3) isoforms of the plasma membrane Ca(2+) pump. Cell Calcium, 2007. PMID: 18029012
- Mahey R, Allen BG, Bridges MA, and Katz S. Regulation of calcium transport in pancreatic acinar plasma membranes from guinea pig. Mol Cell Biochem 112: 155-162, 1992. PMID: 1322490
- Mahey R, Bridges MA, and Katz S. Relationship between Ca(2+)-transport and ATP hydrolytic activities in guinea-pig pancreatic acinar plasma membranes. Mol Cell Biochem 105: 137-147, 1991. PMID: 1833623
- Mankad P, James A, Siriwardena AK, Elliott AC, and Bruce JI. Insulin protects pancreatic acinar cells from cytosolic calcium overload and inhibition of the plasma membrane calcium pump. J Biol Chem 287: 1823-1836, 2012. PMID: 22128146
- Mogami H, Nakano K, Tepikin AV, and Petersen OH. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell 88: 49-55, 1997. PMID: 9019404
- Monteith GR, Wanigasekara Y, and Roufogalis BD. The plasma membrane calcium pump, its role and regulation: new complexities and possibilities. J Pharmacol Toxicol Methods 40: 183-190, 1998. PMID: 10465152
- Muallem S, Beeker T, and Pandol SJ. Role of Na+/Ca2+ exchange and the plasma membrane Ca2+ pump in hormone-mediated Ca2+ efflux from pancreatic acini. J Membr Biol 102: 153-162, 1988. PMID: 2458473
- Neyses L, Reinlib L, and Carafoli E. Phosphorylation of the Ca2+-pumping ATPase of heart sarcolemma and erythrocyte plasma membrane by the cAMP-dependent protein kinase. J Biol Chem 260: 10283-10287, 1985. PMID: 3160706
- Niggli V, Adunyah ES, and Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem 256: 8588-8592, 1981. PMID: 6455424
- Niggli V, Adunyah ES, Penniston JT, and Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem 256: 395-401, 1981. PMID: 6108953
- Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, Alatwi N, Venetucci L, Schuh K, Williams JC, Armesilla AL, and Neyses L. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation 115: 483-492, 2007. PMID: 17242280
- Ollinger K and Brunk UT. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free Radic Biol Med 19: 565-574, 1995. PMID: 8529915
- Ono K, Wang X, and Han J. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol Cell Biol 21: 8276-8288, 2001. PMID: 11713265
- Pandol SJ, Saluja AK, Imrie CW, and Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 133: 1056 e1051-1056 e1025, 2007. PMID: 17383433
- Paszty K, Antalffy G, Hegedus L, Padanyi R, Penheiter AR, Filoteo AG, Penniston JT, and Enyedi A. Cleavage of the plasma membrane Ca+ATPase during apoptosis. Ann N Y Acad Sci 1099: 440-450, 2007. PMID: 17446484
- Paszty K, Antalffy G, Penheiter AR, Homolya L, Padanyi R, Ilias A, Filoteo AG, Penniston JT, and Enyedi A. The caspase-3 cleavage product of the plasma membrane Ca2+-ATPase 4b is activated and appropriately targeted. Biochem J 391: 687-692, 2005. PMID: 16080782
- Paszty K, Verma AK, Padanyi R, Filoteo AG, Penniston JT, and Enyedi A. Plasma membrane Ca2+ATPase isoform 4b is cleaved and activated by caspase-3 during the early phase of apoptosis. J Biol Chem 277: 6822-6829, 2002. PMID: 11751908
- Paul RJ, Hardin CD, Raeymaekers L, Wuytack F, and Casteels R. Preferential support of Ca2+ uptake in smooth muscle plasma membrane vesicles by an endogenous glycolytic cascade. Faseb J 3: 2298-2301, 1989. PMID: 2528493
- Pedersen PL. The cancer cell's "power plants" as promising therapeutic targets: an overview. Journal of bioenergetics and biomembranes 39: 1-12, 2007. PMID: 17404823
- Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. Journal of bioenergetics and biomembranes 39: 211-222, 2007. PMID: 17879147
- Penniston JT and Enyedi A. Modulation of the plasma membrane Ca2+ pump. J Membr Biol 165: 101-109, 1998. PMID: 9744998
- Pottorf WJ, 2nd, Johanns TM, Derrington SM, Strehler EE, Enyedi A, and Thayer SA. Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem 98: 1646-1656, 2006. PMID: 16923173
- Prasad V, Okunade G, Liu L, Paul RJ, and Shull GE. Distinct phenotypes among plasma membrane Ca2+-ATPase knockout mice. Ann N Y Acad Sci 1099: 276-286, 2007. PMID: 17446468
- Puchulu-Campanella E, Chu H, Anstee DJ, Galan JA, Tao WA, and Low PS. Identification of the components of a glycolytic enzyme metabolon on the human red blood cell membrane. J Biol Chem 288: 848-858, 2013. PMID: 23150667
- Ricci JE, Waterhouse N, and Green DR. Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ 10: 488-492, 2003. PMID: 12728246
- Richards DE, Rega AF, and Garrahan PJ. Two classes of site for ATP in the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta 511: 194-201, 1978. PMID: 150288
- Rimessi A, Coletto L, Pinton P, Rizzuto R, Brini M, and Carafoli E. Inhibitory interaction of the 14-3-3{epsilon} protein with isoform 4 of the plasma membrane Ca(2+)-ATPase pump. J Biol Chem 280: 37195-37203, 2005. PMID: 16126729
- Rossi JP and Rega AF. A study to see whether phosphatidylserine, partial proteolysis and EGTA substitute for calmodulin during activation of the Ca2+-ATPase from red cell membranes by ATP. Biochim Biophys Acta 996: 153-159, 1989. PMID: 2526658
- Rossi JP, Villamil AM, Echarte MM, Alzugaray ME, Borelli MI, Garcia ME, Pande J, Grover AK, and Gagliardino JJ. Plasma membrane calcium pump activity in rat pancreatic islets: an accurate method to measure its calcium-dependent modulation. Cell biochemistry and biophysics 46: 193-200, 2006. PMID: 17272847
- Schuh K, Uldrijan S, Gambaryan S, Roethlein N, and Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem 278: 9778-9783, 2003. PMID: 12511555
- Schuh K, Uldrijan S, Telkamp M, Rothlein N, and Neyses L. The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J Cell Biol 155: 201-205, 2001. PMID: 11591728
- Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW, Carafoli E, and Nicotera P. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ 9: 818-831, 2002. PMID: 12107825
- Seigneuret M and Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A 81: 3751-3755, 1984. PMID: 6587389
- Sgambato-Faure V, Xiong Y, Berke JD, Hyman SE, and Strehler EE. The Homer-1 protein Ania-3 interacts with the plasma membrane calcium pump. Biochem Biophys Res Commun 343: 630-637, 2006. PMID: 16554037
- Smallwood JI, Gugi B, and Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J Biol Chem 263: 2195-2202, 1988. PMID: 2963001
- Smriti, Nemergut EC, and Daleke DL. ATP-dependent transport of phosphatidylserine analogues in human erythrocytes. Biochemistry 46: 2249-2259, 2007. PMID: 17269657
- Stauffer TP, Guerini D, and Carafoli E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem 270: 12184-12190, 1995. PMID: 7538133
- Strehler EE and Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev 81: 21-50, 2001. PMID: 11152753
- Szasz I, Sarkadi B, Schubert A, and Gardos G. Effects of lanthanum on calcium-dependent phenomena in human red cells. Biochim Biophys Acta 512: 331-340, 1978. PMID: 152127
- Tepikin AV, Voronina SG, Gallacher DV, and Petersen OH. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J Biol Chem 267: 3569-3572, 1992. PMID: 1310974
- Tepikin AV, Voronina SG, Gallacher DV, and Petersen OH. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem 267: 14073-14076, 1992. PMID: 1629206
- Varadi A, Molnar E, and Ashcroft SJ. A unique combination of plasma membrane Ca2+-ATPase isoforms is expressed in islets of Langerhans and pancreatic beta-cell lines. Biochem J 314 ( Pt 2): 663-669, 1996. PMID: 8670083
- Verma AK, Filoteo AG, Stanford DR, Wieben ED, Penniston JT, Strehler EE, Fischer R, Heim R, Vogel G, Mathews S, and et al. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem 263: 14152-14159, 1988. PMID: 2844759
- Vorherr T, Kessler T, Hofmann F, and Carafoli E. The calmodulin-binding domain mediates the self-association of the plasma membrane Ca2+ pump. J Biol Chem 266: 22-27, 1991. PMID: 1824694
- Warburg O, Wind F, and Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol 8: 519-530, 1927. PMID: 19872213
- Weber H, Huhns S, Luthen F, Jonas L, and Schuff-Werner P. Calpain activation contributes to oxidative stress-induced pancreatic acinar cell injury. Biochem Pharmacol 70: 1241-1252, 2005. PMID: 16154113
- Ximenes HM, Kamagate A, Van Eylen F, Carpinelli A, and Herchuelz A. Opposite effects of glucose on plasma membrane Ca2+-ATPase and Na/Ca exchanger transcription, expression, and activity in rat pancreatic beta-cells. J Biol Chem 278: 22956-22963, 2003. PMID: 12682074
- Zabe M and Dean WL. Plasma membrane Ca(2+)-ATPase associates with the cytoskeleton in activated platelets through a PDZ-binding domain. J Biol Chem 276: 14704-14709, 2001. PMID: 11278574
- Zaidi A and Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca(2+)-ATPase. Free Radic Biol Med 27: 810-821, 1999. PMID: 10515585
- Zhang J, Xiao P, and Zhang X. Phosphatidylserine externalization in caveolae inhibits Ca2+ efflux through plasma membrane Ca2+-ATPase in ECV304. Cell Calcium 45: 177-184, 2009. PMID: 18929409
- Zvaritch E, James P, Vorherr T, Falchetto R, Modyanov N, and Carafoli E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry 29: 8070-8076, 1990. PMID: 2175646
- Zylinska L, Guerini D, Gromadzinska E, and Lachowicz L. Protein kinases A and C phosphorylate purified Ca2+-ATPase from rat cortex, cerebellum and hippocampus. Biochim Biophys Acta 1448: 99-108, 1998. PMID: 9824678


