Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.3
| Attachment | Size |
|---|---|
| 440.35 KB |
Gene symbols: Bhlha15
1. General Function
MIST1 is a transcription factor belonging to the basic helix-loop-helix (bHLH) family of proteins that was discovered using a yeast one hybrid screening approach (13). The Drosophila homolog for MIST1 is dimmed (6) and the gene nomenclature in mice has recently changed from Bhlhb8 (17) to Bhlha15. MIST1 is expressed in serous exocrine cells including acinar cells in the pancreas (16). Outside of the pancreas, MIST1 is expressed in the acinar cells of lacrimal, parotid and submandibular salivary glands, Chief cells of the stomach, alveolar cells of lactating mammary glands, and secreting cells lining the prostate and seminal vesicle (16). In the pancreas, MIST1 localizes to the nuclei of acinar cells (Figure 1A). No expression of MIST1 is observed in duct or centroacinar cells, although recent studies suggest that a population of islet cells may express MIST1 to very low levels (Pin and Konieczny, unpublished).
Functionally, MIST1 is a transcription factor belonging the B family of bHLH proteins, which are members of the family that exhibit a tissue-restricted pattern of expression. The MIST1 protein can form heterodimers with other bHLH transcription factors as is typical for these proteins (12). However, unlike other bHLH proteins, MIST1 appears to preferentially work as a homodimer. This is based on evidence that over-expression of a dominant negative form of MIST1 leads to a similar phenotype in vivo as a targeted ablation of the Mist1 gene (26). A number of genes including Gjb1 (encodes Connexin32; (21), Atp2c2 (encodes SPCA2; (5), p21 CIP1/WAF1 (8), Rab3d (9, 23), Rab26 (23) and PHM (peptidylglycine alpha-hydroxylating monoxygenase; (15) have all been linked to MIST1's transcriptional ability. CAST (Cyclic Amplification and Selection of Targets) experiments have further defined the E box motif that MIST1 binds to as being CATATG (24).
While early studies identified MIST1 as a transcriptional repressor of myogenesis, terming the protein "muscle TWIST" (12), studies in the last 10 years have not identified an in vivo role for MIST1 in myogenesis. There is evidence that MIST1 is a target of the ER stress response transcription factor XBP1 (7), which also plays a physiological role in pancreatic acini. XBP1 promotes Mist1 expression leading to loss of the differentiated state of myoblasts and beta cells in culture (1).
The Mist1 gene is located on chromosome 5 in mus musculus and chromosome 7 in humans (18). The gene consists of two exons with the entire coding region contained in the second exon. The gene encodes for a 197 amino acid protein and, to date, only one isoform and no paralogues are known to exist. Dissection of the MIST1 protein has identified additional motifs outside of the basic helix-loop-helix domain known to allow for DNA binding and dimerization to other bHLH proteins (12, 13). The first 71 amino acids of the protein are believed to contain an active repressor domain based on Gal4 assays with different regions on the protein fused to a GAL4 DNA binding domain. A Q rich region exists in the 3’ end of the protein although a function for this sequence has not been defined. The MIST1 protein does not contain a consensus nuclear localization signal or a transcription activation domain (TAD) and only the bHLH portion of the protein is required for transcriptional activity (24). However, MIST1 is located exclusively in the nucleus and can both promote and repress gene expression following co-transfection with luciferase reporter constructs in cell lines.
2. MIST1 in Pancreas
MIST1 is first expressed in pancreatic development around embryonic day (E) 10.25 (19). Based on this expression pattern it is likely expressed in both exocrine and endocrine precursors. Similar to PTF1a expression, the expression of MIST1 becomes restricted to acinar cells and is not expressed in duct cells or fibroblasts within the pancreas (Figure 1A). MIST1 is localized exclusively to nuclei and is expressed at easily detectable levels in adult tissue (16). The targeted ablation of the Mist1 gene leads to numerous acinar cell phenotypes (19) although the hierarchy of events has still not been adequately established.
The initial study characterizing the phenotype of Mist1-/- mice (19) identified a disruption in acinar cell organization (Figure 1C, E), premature activation of digestive enzymes, increased expression of pancreatitis associated proteins and loss of IP3R3 expression. While Mist1-/- mice are viable with no identified effects on reproduction or overall physiology, the pancreatic phenotype is progressive with increased tissue damage over time and pockets of acinar to duct cell metaplasia appearing in aged mice (19). This metaplasia was also observed in mice that over-express a basic domain mutant of MIST1 that acts as a dominant negative for transcriptional activity (26).
Since the initial study, a number of follow up studies have been published trying to identify a hierarchy of events within the phenotype as well as determine gene targets of MIST1 transcriptional activity. Mist1-/- acini have a defect in mitochondrial localization and calcium movement (14), which is likely the underlying cause of decreased basal and regulated exocytosis exhibited by these cells. In addition to IP3R3, a recent study (5) has identified the specific loss of novel form of Atp2c2 (gene encoding secretory pathway Ca2+ ATPase 2; SPCA2), which has been implicated in store operated calcium entry (4). MIST1 also targets p21 pCIP/WAF causing growth arrest of acinar cells suggesting that, in the absence of MIST1 (8), the mature acinar cell phenotype is more plastic.
One outcome of the phenotypes observed in Mist1-/- mice, is that they are more sensitive to pancreatic injury. A small subset of Mist1-/- animals (<5%) undergoes spontaneous development of pancreatitis, and Mist1-/- mice exhibit significantly more damage and a longer period of recovery following cerulein or arginine-induced pancreatitis (10). Induction of pancreatic injury by cerulein resulted in more necrosis, less apoptosis and higher amounts of circulating amylase compared to wild type counterparts. Microarray analysis revealed marked differences in the molecular profile of Mist1-/- and wild type mice before and after cerulein treatment. In particular, limited activation of the unfolded protein response (UPR), which is robustly observed in wild type tissue (11), is seen in the absence of MIST1. Likely this inability to trigger the UPR is due to an adaptive response of Mist1-/- acini to a stressful cell environment, however, with Mist1 identified as a transcriptional target of XPB1, a direct involvement of MIST1 in the UPR can’t be ruled out. Because of these gaps in knowledge, further work is needed to delineate the targets of MIST1 transcriptional activity.
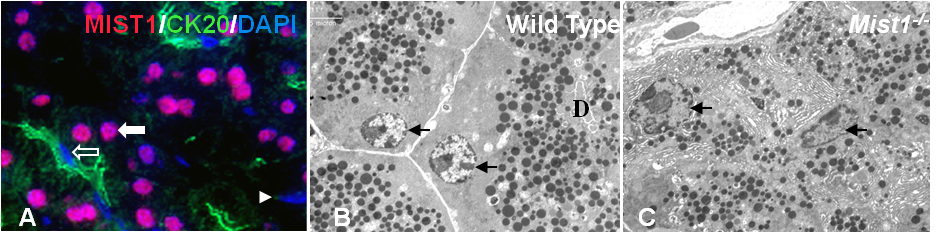
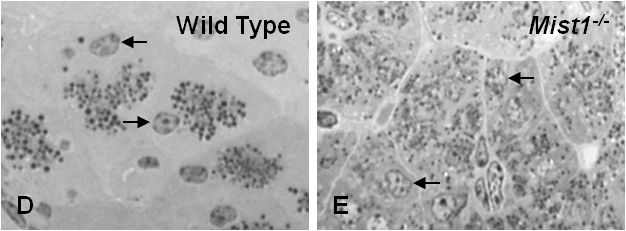
Figure 1. (A) Immunofluorescence for MIST1 (red) and cytokeratin 20 (green) on pancreatic tissue. Acinar cells (white arrow) express MIST1 while duct cells (open arrow) and fibroblasts (arrowhead) do not. (B, C) High magnification electron micrographs and (D, E) methylene blue staining from wild type and Mist1-/- mice. Zymogen granules are dispersed throughout Mist1-/- acini. Nuclei are indicated by black arrows. EMs published in (19).
MIST1 is also expressed in AR42J cells and, to a lesser extent, 266.6 mouse acinar cells, but not in ARIP cells ((16); Pin and Mehmood, unpublished). MIST1 also reduces the ability of oncogenic Kras to promote pancreatic intraepithelial neoplasia (PanIN) (22). The absence of MIST1 in the presence of KrasG12D greatly accelerates PanIN formation and mice are not viable with almost a complete lack of acinar tissue replaced by ductal epithelium. These affects are reversed by the forced expression of MIST1 (22).
An absence of MIST1 also affects the differentiation and/or morphology of other serous exocrine cells including salivary glands (9), gastric epithelium (9, 20), mammary gland alveolar cells (25), seminal vesicle (17), and plasma cells (2, 3).
3. Tools for study of MIST1
a. cDNA clones
cDNA clones for mouse and rabbit MIST1 are available from Stephen Konieczny. A number of DNA constructs were generated in which regions of the MIST1 protein were mutated or deleted affecting MIST1 binding to DNA or other bHLH proteins (12, 24).
b. Rabbit antibodies
Rabbit antibodies raised against the C-terminal of mouse MIST1 have been used to identify MIST1 by western blotting, immunofluorescence and immunohistochemistry in AR42J cells (16), rat (13) and pancreas (19). Additional antibodies have been made available by Santa Cruz Biotechnology as a IgG and stated to detect all isoforms of MIST1 of mouse, rat, and human origin. While these antibodies are believed to be ChIP ready, they have shown limited success in this assay to date.
c. Mouse lines
There are at least four reported transgenic and gene targeting mice that affect the expression of MIST1. Targeted ablation of the Mist1 gene has been reported in which the Mist1 coding region has been replaced by the LacZ reporter gene (19). A subsequent targeting event replaced the Mist1 coding region with cre recombinase, thereby providing the ability to specifically delete floxed alleles in MIST1-expressing cells (22). This has allowed for lineage tracing of cells that express MIST1 transiently. A dominant-negative form of MIST1 in which the basic region was mutated to prevent DNA binding was expressed specifically in pancreatic acini using the Elastase I promoter leading to similar acinar cell disorganization as described in Mist1-/- mice (26). Finally, a transgenic mice which allows for inducible expression of a myc-tagged MIST1 protein has been generated (5). These lines are all available from Stephen Konieczny.
Acknowledgements
Grant funding provided by the Canadian Institutes of Health Research (CIHR; CP - MOP 53083), Natural Sciences and Engineering Research Council, Children's Health Research Institute, and Lawson Health Research Institute.
4. References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, and Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Molecular cell 27: 53-66, 2007. PMID:17612490
- Bhattacharya D, Cheah MT, Franco CB, Hosen N, Pin CL, Sha WC, and Weissman IL. Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J Immunol 179: 6808-6819, 2007. PMID:17982071
- Capoccia BJ, Lennerz JK, Bredemeyer AJ, Klco JM, Frater JL, and Mills JC. The transcription factor MIST1 in terminal differentiation of mouse and human plasma cells. Physiological genomics, 2010. PMID:21098683
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, and Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 143: 84-98, 2010. PMID:20887894
- Garside VC, Kowalik AS, Johnson CL, DiRenzo D, Konieczny SF, and Pin CL. MIST1 regulates the pancreatic acinar cell expression of Atp2c2, the gene encoding secretory pathway calcium ATPase 2. Experimental cell research 316: 2859-2870, 2010. PMID:20599950
- Hewes RS, Park D, Gauthier SA, Schaefer AM, and Taghert PH. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development (Cambridge, England) 130: 1771-1781, 2003. PMID:12642483
- Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, and Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139: 2038-2049, 2010. PMID:20816838
- Jia D, Sun Y, and Konieczny SF. Mist1 regulates pancreatic acinar cell proliferation through p21 CIP1/WAF1. Gastroenterology 135: 1687-1697, 2008. PMID:18762186
- Johnson CL, Kowalik AS, Rajakumar N, and Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mechanisms of development 121: 261-272, 2004. PMID:15003629
- Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, and Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. American journal of physiology 292: G1123-1132, 2007. PMID:17170023
- Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, and Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. American journal of physiology 291: G238-245, 2006. PMID:16574987
- Lemercier C, To RQ, Carrasco RA, and Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. The EMBO journal 17: 1412-1422, 1998. PMID:9482738
- Lemercier C, To RQ, Swanson BJ, Lyons GE, and Konieczny SF. Mist1: a novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Developmental biology 182: 101-113, 1997. PMID:9073453
- Luo X, Shin DM, Wang X, Konieczny SF, and Muallem S. Aberrant localization of intracellular organelles, Ca2+ signaling, and exocytosis in Mist1 null mice. The Journal of biological chemistry 280: 12668-12675, 2005. PMID:15665001
- Park D, Shafer OT, Shepherd SP, Suh H, Trigg JS, and Taghert PH. The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Molecular and cellular biology 28: 410-421, 2008. PMID:17967878
- Pin CL, Bonvissuto AC, and Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. The Anatomical record 259: 157-167, 2000. PMID:10820318
- Pin CL, Johnson CL, Rade B, Kowalik AS, Garside VC, and Everest ME. Identification of a transcription factor, BHLHB8, involved in mouse seminal vesicle epithelium differentiation and function. Biology of reproduction 78: 91-100, 2008. PMID:17901072
- Pin CL, Lemercier C, and Konieczny SF. Cloning of the murine Mist1 gene and assignment to mouse chromosome band 5G2-5G3. Cytogenetics and cell genetics 86: 219-222, 1999. PMID:10575209
- Pin CL, Rukstalis JM, Johnson C, and Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. The Journal of cell biology 155: 519-530, 2001. PMID:11696558
- Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, and Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development (Cambridge, England) 134: 211-222, 2007. PMID:17164426
- Rukstalis JM, Kowalik A, Zhu L, Lidington D, Pin CL, and Konieczny SF. Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1. Journal of cell science 116: 3315-3325, 2003. PMID:12829745
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, and Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 136: 1368-1378, 2009. PMID:19249398
- Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Blazewska KM, McKenna CE, and Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Molecular and cellular biology 30: 1269-1284, 2010. PMID:20038531
- Tran T, Jia D, Sun Y, and Konieczny SF. The bHLH domain of Mistl is sufficient to activate gene transcription. Gene expression 13: 241-253, 2007. PMID:17605298
- Zhao Y, Johansson C, Tran T, Bettencourt R, Itahana Y, Desprez PY, and Konieczny SF. Identification of a basic helix-loop-helix transcription factor expressed in mammary gland alveolar cells and required for maintenance of the differentiated state. Molecular endocrinology (Baltimore, Md 20: 2187-2198, 2006. PMID:16645041
- Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, and Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Molecular and cellular biology 24: 2673-2681, 2004. PMID:15024058


