Entry Version:
Citation:
Pancreapedia: Exocrine Pancreas Knowledge Base, DOI: 10.3998/panc.2011.31
| Attachment | Size |
|---|---|
| 186.33 KB |
Official Symbol: Cplx2
1. General function
Complexins are a small family of proteins comprised of 4 members – complexin 1-4. Complexin 1, originally identified as synaphin, is a small 134 amino acid cytosolic protein that is expressed exclusively in neurons where it associates with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes necessary for exocytosis (16,25). Complexin 2 is ubiquitously expressed and thought to function in an analogous manner in other secretory cells. Complexin 1 and 2 are 86% identical at the amino acid level (2). Moreover, despite considerable differences in their mRNA sequences, mouse, rat and human complexin 2 proteins are 100% identical (20). Complexin 3 and 4 were recently identified and evidence of their specific function is still unclear. Complexin 3 is expressed strongly in retina and certain regions of the brain including the hippocampus and thalamus, whereas complexin 4 is only expressed in retina (24).
Complexin 1 was originally proposed to clamp ternary SNARE complexes composed of vesicle associated membrane protein (VAMP) 2 on synaptic vesicles and syntaxin 1 and SNAP 25 on the plasma membrane at a pre-fusion step in the exocytic process thereby inhibiting neurotransmitter secretion (8,31). It was proposed that upon elevation of intracellular Ca2+, complexin 1 is displaced from the SNARE complex by the synaptic vesicle protein synaptotagmin 1, in turn, allowing the final stages of neurotransmitter release to commence (8). More recent studies have shown that in addition to this clamping activity, complexin 1 also directly modulates the final stages of SNARE complex formation to facilitate exocytosis (27,29).
Complexins are comprised of an N-terminal region (aa 1-29), accessory alpha-helix (aa 30-48), central alpha-helix (aa 49-70) and C-terminal region (aa 71-134) (Fig 1). In vitro vesicle fusion assays and mutational analysis in neurons indicate the N-terminus has a positive regulatory effect on vesicle fusion and activates synchronous Ca2+-induced exocytosis (19,30). Conversely, the accessory alpha helix has a negative regulatory effect on vesicle fusion and restricts spontaneous exocytosis (19,30). The central alpha helix is essential for SNARE binding, whereas the function of the C-terminus is unclear (30).
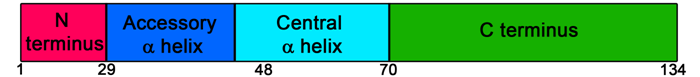
Figure 1. Schematic of complexin 1 and 2 functional domains. See text for details
Complexin 1 binds with high affinity to the ternary SNARE complex and with lower affinity to the t-SNARE complex containing SNAP 25 and syntaxin 1 yet is unable to bind monomeric SNARE proteins (13,17,20,21,29). Complexin 1 binds the ternary SNARE complex as an antiparallel helix in the groove between the VAMP 2 and syntaxin helices (4,15,19,20,22,30). Complexin 1 has also been shown to function in neurons by simultaneously suppressing spontaneous vesicle fusion and activating fast Ca2+-stimulated fusion (19). Additional evidence demonstrates that complexin 1 binding controls the force that trans-SNARE complexes apply onto opposing membranes to facilitate their fusion (19).
Complexin 2 has been studied in neural, chromaffin, mast and pancreatic acinar cells all of which support a functional role in Ca2+-triggered exocytosis (3,6,18,26,27). Knockdown of complexin 2 expression in RBL-2H3 mast cells attenuated Ca2+-dependent degranulation (27).
2. Specific function in exocrine pancreas
Expression of complexin 2 in rat pancreatic acinar cells was demonstrated by reverse transcription PCR, immunoblotting and immunofluorescence microscopy (6). Complexin 2 is a cytosolic protein that is not found on zymogen granule membranes but does accumulate along the apical membrane. GST-pulldown assays in acinar lysates demonstrate that complexin 2 interacts with VAMP 2, syntaxin 3 and syntaxin 4 but not VAMP8. Furthermore, complexin 2 colocalizes with VAMP 2 along the apical membrane and this association is significantly enhanced following CCK-8 stimulation (Fig 2).
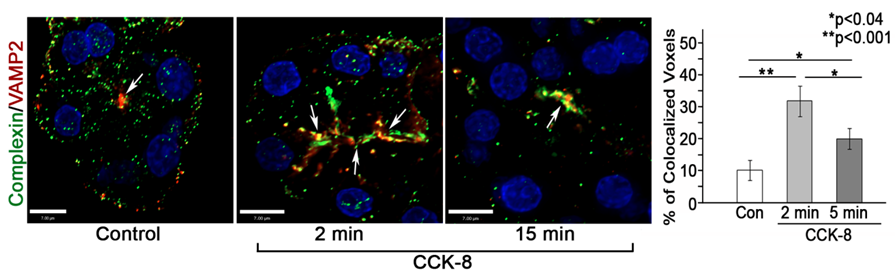
Figure 2. Colocalization of complexin 2 and VAMP2 in apical membrane regions of pancreatic acini. Left and middle panels demonstrate the enhanced accumulation of complexin 2 at the apical plasma membrane following CCK-8 (100 pM) stimulation. Right panel shows quantification of multiple reconstructed z-series images from 3 separate tissue preparations of the % of total complexin 2 colocalized with VAMP2 at the plasma membrane. This data is reproduced from ref 6.
A functional role for complexin 2 in exocrine secretion was demonstrated by introducing recombinant wild-type and mutated forms of the protein into perfringolysin-O permeabilized acinar cells. High concentrations of recombinant complexin 2 inhibited Ca2+-dependent amylase release and these effects were abolished by mutations that inhibited complexin 2 interactions with VAMP 2 (6). These results are consistent with studies examining complexin 1 exocytic function in neurons and suggest that high levels of complexin 2 stabilize trans-SNARE complex formation at a prefusion state, thereby inhibiting Ca2+-stimulated secretion.
3. Tools to study complexin
a. Antibodies
Purified rabbit anti-complexin 1,2 antibodies (Synaptic Systems cat # 122 002 and 122 102) were successfully used in rat pancreas for immunoblotting and immunofluorescence (1,5,6).
b. cDNA clones
In 1995, McMahon and colleagues cloned in rat brain, complexin 1 and 2 and inserted full length complexin 1 or complexin 2 into pGEX-KG (20). Since then several mutations and truncations of complexin 1 have been produced and studied in brain (9,18,19,30).
c. Knockout mice
Complexin 1/2 double knockout mice die shortly after birth (23). However, single knockout mice however are viable.
Complexin 1 knockout mice are infertile (32). They are also characterized by abnormal early motor development, reduced exploratory behavior, profound ataxia, deficits in social behavior and reduced habitation yet have normal cognitive function (10,11,12,23).
Complexin 2 knockout mice show no overt phenotypic changes (23), yet they have cognitive and motor deficits (10). In the CA1 and CA3 regions of the hippocampus, complexin 2 knockout mice show altered long-term synaptic potentiation (7,14,28). However, synaptic transmission is not altered in the hippocampus of these mice (14).
d. siRNA
Complexin 2-specific siRNA are available from Santa Cruz (sc-41925, sc-41926).
4. References
- Abderrahmani A, Niederhauser G, Plaisance V, Roehrich ME, Lenain V, Coppola T, Regazzi R and Waeber G. Complexin I regulates glucose-induced secretion in pancreatic beta-cells. J.Cell.Sci. 117: Pt 11: 2239-2247, 2004. PMID: 15126625
- Brose N. For better or for worse: complexins regulate SNARE function and vesicle fusion. Traffic 9: 9: 1403-1413, 2008. PMID: 18445121
- Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sorensen JB, Brose N and Chow RH. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc.Natl.Acad.Sci.U.S.A. 105: 49: 19538-19543, 2008. PMID: 19033464
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC and Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron 33: 3: 397-409, 2002. PMID: 11832227
- Dubois M, Vacher P, Roger B, Huyghe D, Vandewalle B, Kerr-Conte J, Pattou F, Moustaid-Moussa N and Lang J. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology 148: 4: 1605-1614, 2007. PMID: 17204559
- Falkowski MA, Thomas DD and Groblewski GE. Complexin 2 modulates vesicle-associated membrane protein (VAMP) 2-regulated zymogen granule exocytosis in pancreatic acini. J.Biol.Chem. 285: 46: 35558-35566, 2010. PMID: 20829354
- Gibson HE, Reim K, Brose N, Morton AJ and Jones S. A similar impairment in CA3 mossy fibre LTP in the R6/2 mouse model of Huntington's disease and in the complexin II knockout mouse. Eur.J.Neurosci. 22: 7: 1701-1712, 2005. PMID: 16197510
- Giraudo CG, Eng WS, Melia TJ and Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science 313: 5787: 676-680, 2006. PMID: 16794037
- Giraudo CG, Garcia-Diaz A, Eng WS, Yamamoto A, Melia TJ and Rothman JE. Distinct domains of complexins bind SNARE complexes and clamp fusion in vitro. J.Biol.Chem. 283: 30: 21211-21219, 2008. PMID: 18499660
- Glynn D, Bortnick RA and Morton AJ. Complexin II is essential for normal neurological function in mice. Hum.Mol.Genet. 12: 19: 2431-2448, 2003. PMID: 12915444
- Glynn D, Drew CJ, Reim K, Brose N and Morton AJ. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum.Mol.Genet. 14: 16: 2369-2385, 2005. PMID: 16000319
- Glynn D, Sizemore RJ and Morton AJ. Early motor development is abnormal in complexin 1 knockout mice. Neurobiol.Dis. 25: 3: 483-495, 2007. PMID: 17188502
- Hu K, Carroll J, Rickman C and Davletov B. Action of complexin on SNARE complex. J.Biol.Chem. 277: 44: 41652-41656, 2002. PMID: 12200427
- Huang GZ, Ujihara H, Takahashi S, Kaba H, Yagi T and Inoue S. Involvement of complexin II in synaptic plasticity in the CA1 region of the hippocampus: the use of complexin II-lacking mice. Jpn.J.Pharmacol. 84: 2: 179-187, 2000. PMID: 11128041
- Ishizuka T, Saisu H, Odani S and Abe T. Synaphin: a protein associated with the docking/fusion complex in presynaptic terminals. Biochem.Biophys.Res.Commun. 213: 3: 1107-1114, 1995. PMID: 7654227
- Li L and Chin LS. The molecular machinery of synaptic vesicle exocytosis. Cell Mol.Life Sci. 60: 5: 942-960, 2003. PMID: 12827282
- Liu J, Guo T, Wei Y, Liu M and Sui SF. Complexin is able to bind to SNARE core complexes in different assembled states with distinct affinity. Biochem.Biophys.Res.Commun. 347: 2: 413-419, 2006. PMID: 16828463
- Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM and Sollner TH. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc.Natl.Acad.Sci.U.S.A. 106: 6: 2001-2006, 2009. PMID: 19179400
- Maximov A, Tang J, Yang X, Pang ZP and Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323: 5913: 516-521, 2009. PMID: 19164751
- McMahon HT, Missler M, Li C and Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83: 1: 111-119, 1995. PMID: 7553862
- Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J and Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J.Biol.Chem. 275: 26: 19808-19818, 2000. PMID: 10777504
- Pabst S, Margittai M, Vainius D, Langen R, Jahn R and Fasshauer D. Rapid and selective binding to the synaptic SNARE complex suggests a modulatory role of complexins in neuroexocytosis. J.Biol.Chem. 277: 10: 7838-7848, 2002. PMID: 11751907
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N and Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104: 1: 71-81, 2001. PMID: 11163241
- Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K and Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J.Cell Biol. 169: 4: 669-680, 2005. PMID: 15911881
- Sollner TH. Regulated exocytosis and SNARE function (Review). Mol.Membr.Biol. 20: 3: 209-220, 2003. PMID: 12893529
- Tadokoro S, Nakanishi M and Hirashima N. Complexin II regulates degranulation in RBL-2H3 cells by interacting with SNARE complex containing syntaxin-3. Cell.Immunol. 261: 1: 51-56, 2010. PMID: 19932892
- Tadokoro S, Nakanishi M and Hirashima N. Complexin II facilitates exocytotic release in mast cells by enhancing Ca2+ sensitivity of the fusion process. J.Cell.Sci. 118: Pt 10: 2239-2246, 2005. PMID: 15870114
- Takahashi S, Ujihara H, Huang GZ, Yagyu KI, Sanbo M, Kaba H and Yagi T. Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur.J.Neurosci. 11: 7: 2359-2366, 1999. PMID: 10383625
- Tokumaru H, Umayahara K, Pellegrini LL, Ishizuka T, Saisu H, Betz H, Augustine GJ and Abe T. SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell 104: 3: 421-432, 2001. PMID: 11239399
- Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, Brose N and Rosenmund C. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat.Struct.Mol.Biol. 14: 10: 949-958, 2007. PMID: 17828276
- Yoon TY, Lu X, Diao J, Lee SM, Ha T and Shin YK. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat.Struct.Mol.Biol. 15: 7: 707-713, 2008. PMID: 18552825
- Zhao L, Burkin HR, Shi X, Li L, Reim K and Miller DJ. Complexin I is required for mammalian sperm acrosomal exocytosis. Dev.Biol. 309: 2: 236-244, 2007. PMID: 17692307


